The Occurrence of Illicit Smart Drugs or Nootropics in Europe and Australia and Their Associated Dangers: Results from a Market Surveillance Study by 12 Official Medicines Control Laboratories
Abstract
1. Introduction
2. Materials and Methods
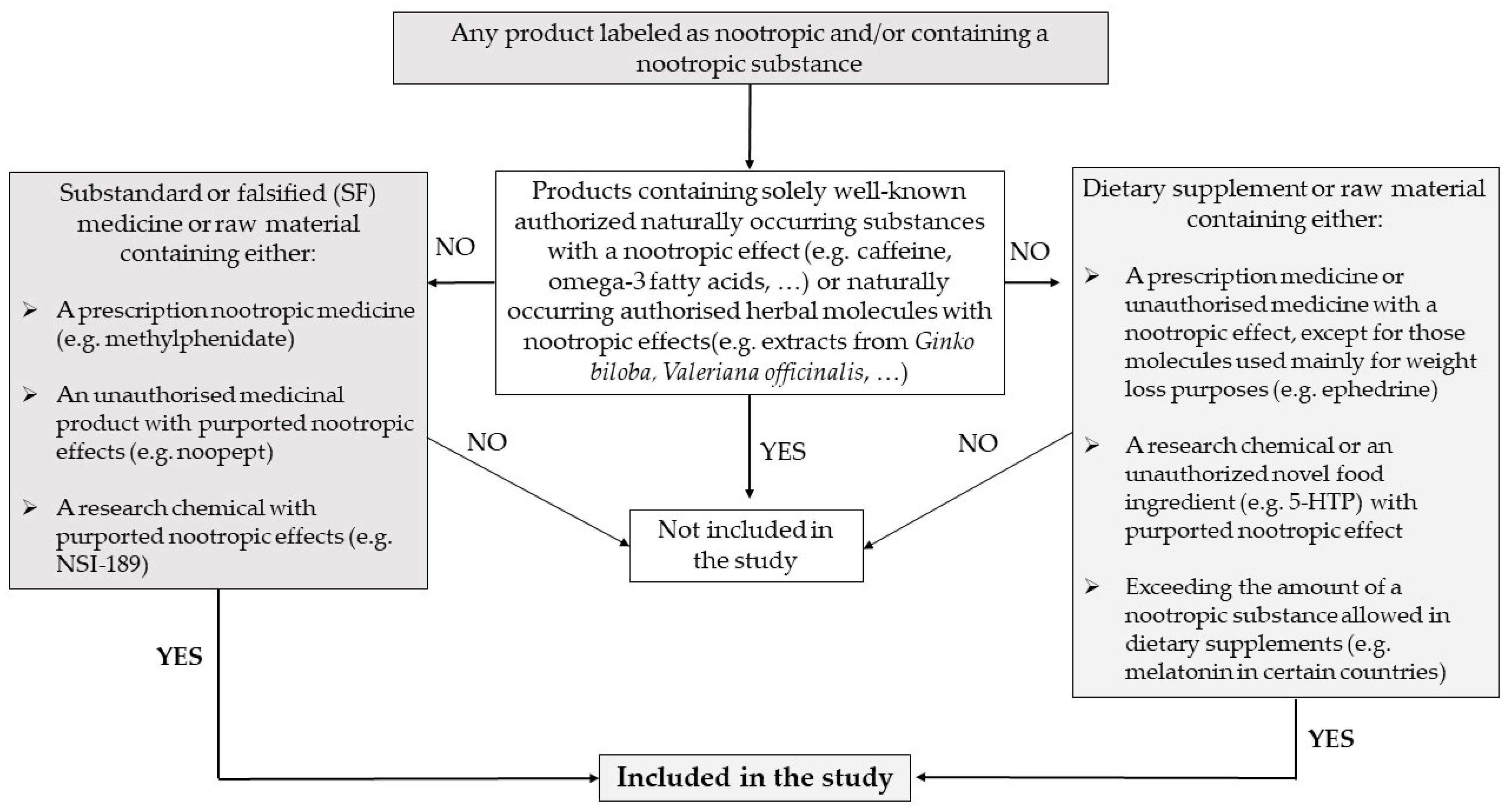
2.1. Scope
2.2. Data Collection and Time Frame
3. Results
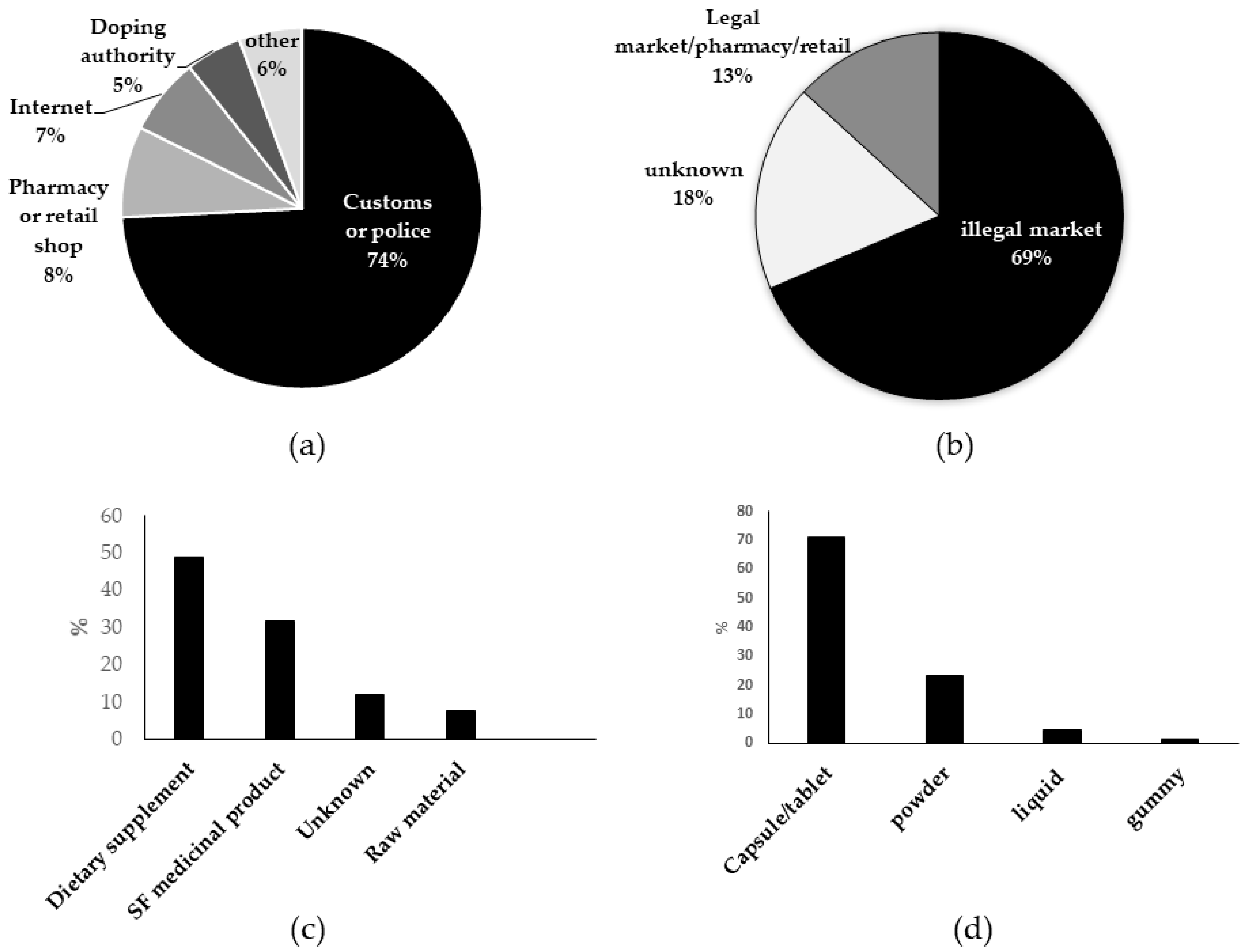
3.1. Origin of the Samples
3.2. Product Type and Dosage Form of Tested Samples
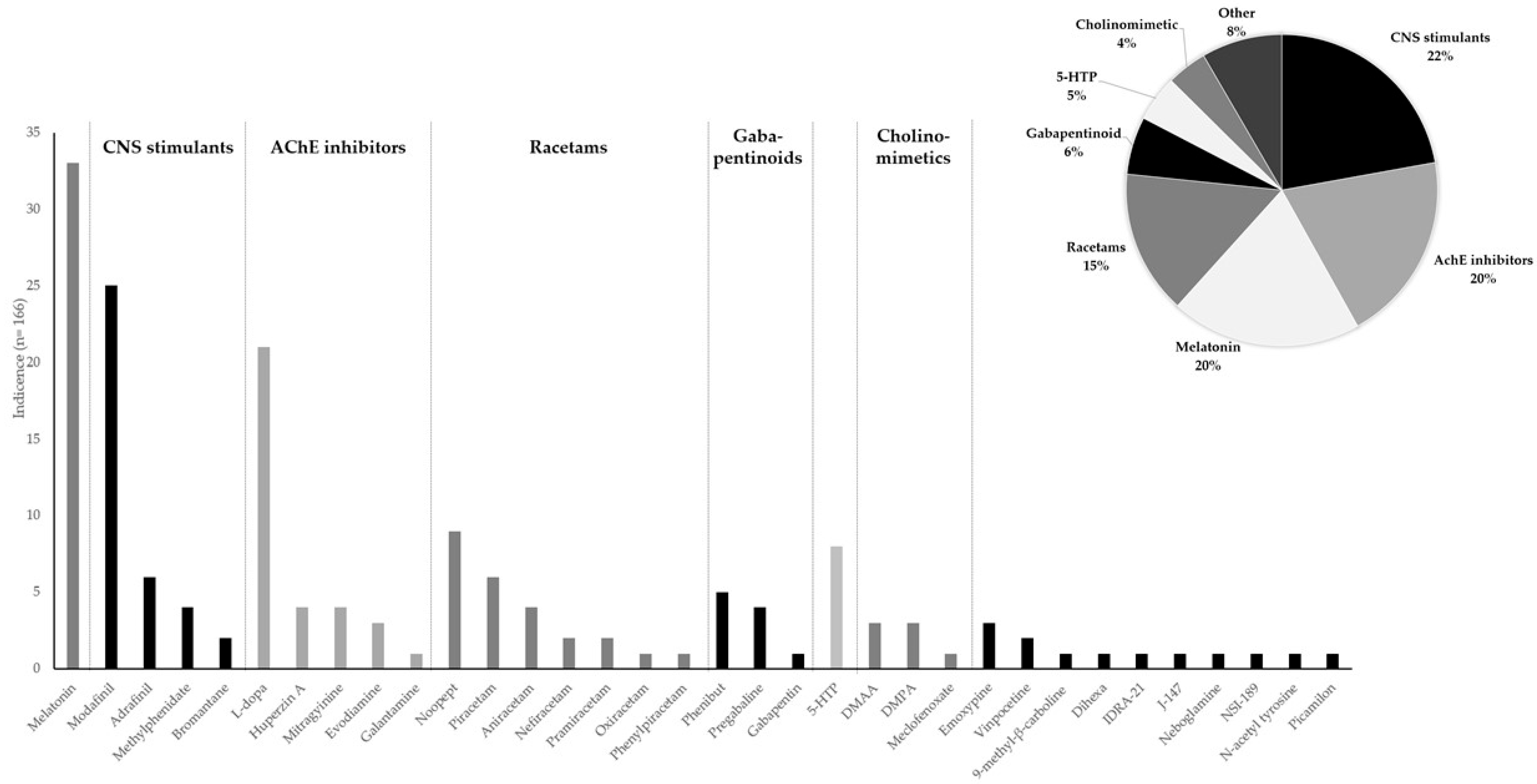
3.3. Detected Molecules and Their Quantities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Molecule Found | Detection Frequency | # Laboratories Where Detected | Mainly Presented as | Legal Status |
|---|---|---|---|---|
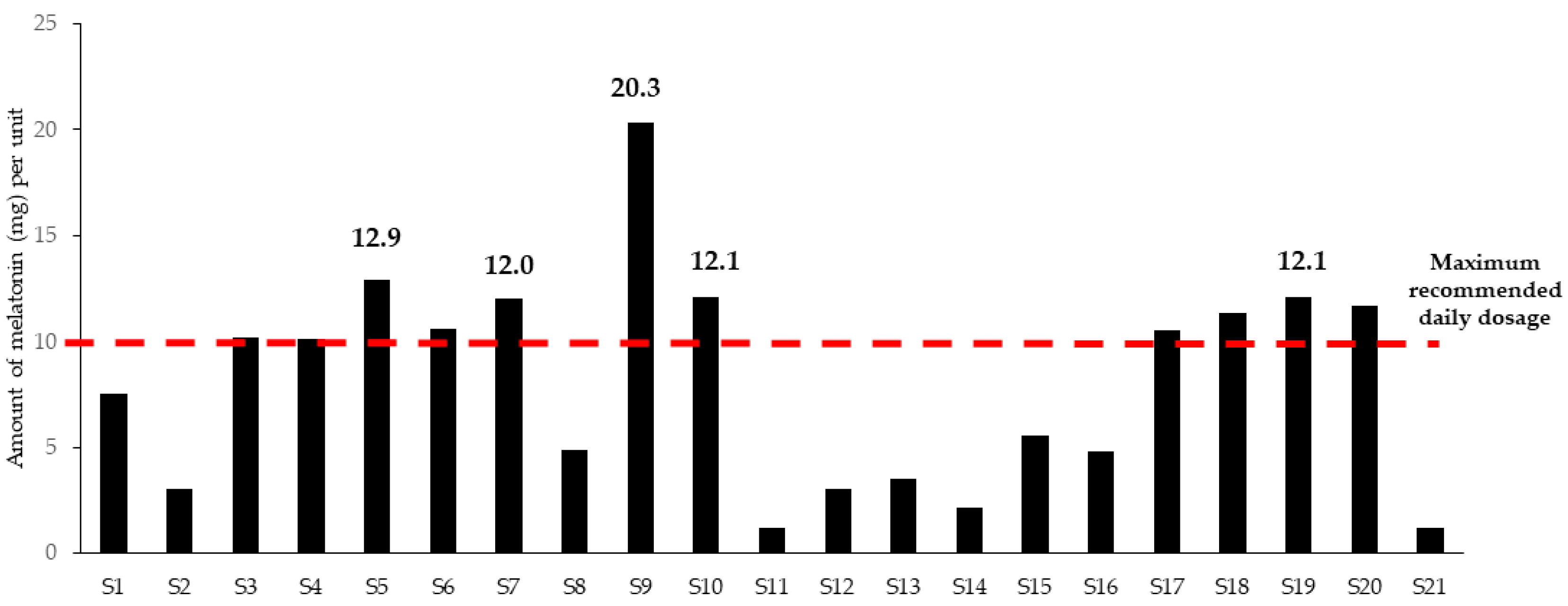
| Melatonin | 33 | 7 | DS | Melatonin regulation varies. In Australia and some EU countries, it is a prescription drug and thus not allowed in dietary supplements. The limit for dietary supplements containing melatonin is 0.3 mg per day in the Netherlands, Luxembourg, and Belgium, 1 mg in Italy, Spain, and Poland, and 2 mg in France. In Germany, the situation is unclear and depends on the court. |
| Modafinil | 25 | 6 | SF medicine and DS | Prescription medicine in the EU and Australia. |
| L-dopa | 21 | 4 | DS | Prescription medicine in the EU and Australia. The molecule is also present in the plant Macuna pruriens, an unauthorised novel food in the EU. In Australia, Mucuna pruriens is a permitted ingredient with L-dopa as a mandatory component with an allowed limit of 1 mg/kg or 1 mg/L. |
| Noopept | 9 | 4 | SF medicine and DS | Not authorised for human use by any health authority in the countries where the molecule was found. |
| 5-HTP | 8 | 4 | DS | Unauthorised novel food in the EU. In Australia, 5-HTP is regarded as a derivative of the prescription medicine tryptophan. Any product for human therapeutic use with a label claim of greater than 100 mg 5-HTP per recommended daily dose would be regarded as a prescription medicine. |
| Adrafinil | 6 | 2 | SF medicine and DS | Discontinued medicinal product, formerly a prescription medicine (prodrug of modafinil). |
| Piracetam | 6 | 3 | SF medicine and DS | Prescription medicine in the EU and Australia. |
| Phenibut | 5 | 4 | DS | Not authorised for human use by any health authority in the EU or Australia, registered as a controlled substance. |
| Aniracetam | 4 | 3 | SF medicine | Prescription medicine in the EU and Australia. |
| Huperzine A | 4 | 2 | DS | Huperzine A is extracted from Huperzia serrata and is considered an unauthorised novel food in many EU member states, with some exceptions (e.g., Belgium, France and Romania). It is not approved for use in medicines in Australia. |
| Methylphenidate | 4 | 1 | SF medicine | Prescription medicine in the EU and Australia |
| Mitragynine | 4 | 4 | SF medicine and DS | Found in Mitragyna speciosa. The plant is considered an unauthorised novel food in the EU. In some EU member states and Australia, it is considered a controlled substance |
| Pregabaline | 4 | 1 | SF medicine | Prescription medicine in the EU and Australia |
| Emoxypine | 3 | 2 | SF medicine and DS | Research chemical for which little reliable clinical information is available, thus not authorised for human consumption in the EU or Australia. |
| DMAA | 3 | 3 | DS | Banned in dietary supplements in the EU and Australia |
| DMPA | 3 | 2 | DS | Not authorised in dietary supplements in the EU or Australia |
| Evodiamine | 3 | 1 | DS | Research chemical. The molecule can be extracted from the dried, nearly ripe fruit of Evodia rutaecarpa, an unauthorised novel food in the EU and not approved for use in Australia. |
| Bromantane | 2 | 2 | unknown | Research chemical in the EU and Australia, used as a prescription drug in Russia |
| Nefiracetam | 2 | 1 | SF medicine | Piracetam homologue, not authorised for human use by any health authority in the EU or Australia |
| Pramiracetam | 2 | 1 | SF medicine | Piracetam homologue, not authorised for human use by any health authority in the EU or Australia |
| Vinpocetine | 2 | 2 | SF medicine | Prescription medicine in certain EU member states (e.g., Poland and Germany), unauthorised in others and in Australia |
| 9-MBC | 1 | 1 | unknown | Research chemical |
| Meclofenoxate | 1 | 1 | unknown | Prescription medicine in some EU member states (e.g., Austria and Germany) and in Australia, not approved in others |
| Dihexa | 1 | 1 | DS | Research chemical |
| Gabapentin | 1 | 1 | SF medicine | Prescription medicine in the EU and Australia |
| Galantamine | 1 | 1 | Unknown | Prescription medicine in the EU and Australia |
| IDRA-21 | 1 | 1 | Unknown | Research chemical |
| J-147 | 1 | 1 | Unknown | Research chemical |
| N-acetyl tyrosine | 1 | 1 | DS | Unauthorised novel food ingredient |
| Neboglamine | 1 | 1 | SF medicine | Research chemical |
| NSI-189 | 1 | 1 | Unknown | Research chemical |
| Oxiracetam | 1 | 1 | Unknown | Piracetam homologue, not authorised for human use by any health authority in the EU or Australia |
| Phenylpiracetam | 1 | 1 | SF medicine | Piracetam homologue and not authorised for human use by any health authority in the EU or Australia |
| Picamilon | 1 | 1 | DS | Research chemical in the EU and Australia, used as a prescription drug in Russia |
References
- Lucke, J.C.; Bell, S.K.; Partridge, B.J.; Hall, W.D. Academic doping or Viagra for the brain? The history of recreational drug use and pharmacological enhancement can provide insight into these uses of neuropharmaceuticals. EMBO Rep. 2011, 12, 197–201. [Google Scholar] [CrossRef]
- Maier, L.J.; Ferris, J.A.; Winstock, A.R. Pharmacological cognitive enhancement among non-ADHD individuals-A cross-sectional study in 15 countries. Int. J. Drug Policy 2018, 58, 104–112. [Google Scholar] [CrossRef]
- Franke, A.G.; Koller, G.; Krause, D.; Proebstl, L.; Kamp, F.; Pogarell, O.; Jebrini, T.; Manz, K.; Chrobok, A.I.; Soyka, M. Just “Like Coffee” or Neuroenhancement by Stimulants? Front. Public Health 2021, 9, 640154. [Google Scholar] [CrossRef]
- Malík, M.; Tlustoš, P. Nootropics as Cognitive Enhancers: Types, Dosage and Side Effects of Smart Drugs. Nutrients 2022, 14, 3367. [Google Scholar] [CrossRef]
- Jędrejko, K.; Catlin, O.; Stewart, T.; Anderson, A.; Muszyńska, B.; Catlin, D.H. Unauthorized ingredients in “nootropic” dietary supplements: A review of the history, pharmacology, prevalence, international regulations, and potential as doping agents. Drug Test. Anal. 2023, 15, 803–839. [Google Scholar] [CrossRef]
- Vanhee, C.; Tuenter, E.; Kamugisha, A.; Canfyn, M.; Moens, G.; Courselle, P.; Pieters, L.; Deconinck, E.; Exarchou, V. Identification and Quantification Methodology for the Analysis of Suspected Illegal Dietary Supplements: Reference Standard or no Reference Standard, that’s the Question. J. Forensic Toxicol. Pharmacol. 2018, 7, 1. [Google Scholar] [CrossRef]
- Cohen, P.A.; Avula, B.; Wang, Y.H.; Zakharevich, I.; Khan, I. Five Unapproved Drugs Found in Cognitive Enhancement Supplements. Neurol. Clin. Pract. 2021, 11, e303–e307. [Google Scholar] [CrossRef]
- Cohen, P.A.; Ellison, R.R.; Travis, J.C.; Gaufberg, S.V.; Gerona, R. Quantity of phenibut in dietary supplements before and after FDA warnings. Clin. Toxicol. 2022, 60, 486–488. [Google Scholar] [CrossRef]
- Cohen, P.A.; Avula, B.; Katragunta, K.; Khan, I. Levodopa Content of Mucuna pruriens Supplements in the NIH Dietary Supplement Label Database. JAMA Neurol. 2022, 79, 1085–1086. [Google Scholar] [CrossRef]
- Cohen, P.A.; Avula, B.; Khan, I. The unapproved drug centrophenoxine (meclofenoxate) in cognitive enhancement dietary supplements. Clin Toxicol. 2022, 60, 1156–1158. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jacobs, B.; Van Hoorde, K.; Vanhee, C. Accuracy of Labeling of Galantamine Generic Drugs and Dietary Supplements. JAMA 2024, 331, 974–976. [Google Scholar] [CrossRef]
- Paiva, R.; Correia, M.; Delerue-Matos, C.; Amaral, J.S. Adulteration of Brain Health (Cognitive, Mood, and Sleep Enhancement) Food Supplements by the Addition of Pharmaceutical Drugs: A Comprehensive Review of Analytical Approaches and Trends. Foods 2024, 13, 908. [Google Scholar] [CrossRef]
- Sahakian, B.; Morein-Zamir, S. Professor’s little helper. Nature 2007, 450, 1157–1159. [Google Scholar] [CrossRef]
- Bogle, K.E.; Smith, B.H. Illicit methylphenidate use: A review of prevalence, availability, pharmacology, and consequences. Curr. Drug Abus. Rev. 2009, 2, 157–176. [Google Scholar] [CrossRef]
- Wilms, W.; Woźniak-Karczewska, M.; Corvini, P.F.; Chrzanowski, Ł. Nootropic drugs: Methylphenidate, modafinil and piracetam—Population use trends, occurrence in the environment, ecotoxicity and removal methods—A review. Chemosphere 2019, 233, 771–785. [Google Scholar] [CrossRef]
- Deconinck, E.; Vanhee, C.; Keizers, P.; Guinot, P.; Mihailova, A.; Syversen, P.V.; Li-Ship, G.; Young, S.; Blazewicz, A.; Poplawska, M.; et al. The occurrence of non-anatomical therapeutic chemical-international nonproprietary name molecules in suspected illegal or illegally traded health products in Europe: A retrospective and prospective study. Drug Test. Anal. 2021, 13, 833–840. [Google Scholar] [CrossRef]
- European Commision, Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2002:031:TOC (accessed on 10 April 2025).
- European Commission Regulation (EU) 2015/403 of 11 March 2015 Amending Annex IIIto Regulation (EC) No 1925/2006 of the European Parliament of the Council as Regards Ephedra Species Yohimbe (Pausinystalia yohimbe, (K. Schum) Pierre ex Beille). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2015:067:TOC (accessed on 10 April 2025).
- European Commission, Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2015:327:TOC (accessed on 10 April 2025).
- Food Standards Australia and New Zealand, Novel Foods. Available online: https://www.foodstandards.gov.au/business/novel (accessed on 10 April 2025).
- European Directorate for the Quality of Medicines & Healthcare, Testing of Falsified/Illegal Medicines, Study Report on Slimming Dietary Supplements. Available online: https://www.edqm.eu/en/testing-of-falsified-/-illegal-medicines (accessed on 10 April 2025).
- European Directorate for the Quality of Medicines & Healthcare, Testing of Falsified/Illegal Medicines, Study Report on Dietary Supplements Advertised as Sexual Potency Enhancers. Available online: https://www.edqm.eu/en/testing-of-falsified-/-illegal-medicines (accessed on 10 April 2025).
- European Directorate for the Quality of Medicines & Healthcare, Testing of Falsified/Illegal Medicines, Study Report on Illegal Anabolic Steroids. Available online: https://www.edqm.eu/en/testing-of-falsified-/-illegal-medicines (accessed on 10 April 2025).
- European Directorate for the Quality of Medicines & Healthcare, Testing of Falsified/Illegal Medicines, Study Report on Medicines in Disguise. Available online: https://www.edqm.eu/en/testing-of-falsified-/-illegal-medicines (accessed on 10 April 2025).
- European Directorate for the Quality of Medicines & Healthcare, Testing of Falsified/Illegal Medicines, Study Report on Illegal Products Containing “Non-INN” APIs. Available online: https://www.edqm.eu/en/testing-of-falsified-/-illegal-medicines (accessed on 10 April 2025).
- European Directorate for the Quality of Medicines & Healthcare, Testing of Falsified/Illegal Medicines, Study Report on SARMs, Metabolic Modulators and Small Molecule Growth Hormone Secretagogues Used as Performance Enhancing Substances. Available online: https://www.edqm.eu/en/testing-of-falsified-/-illegal-medicines (accessed on 10 April 2025).
- European Directorate for the Quality of Medicines & Healthcare, Annex3, List of GEON Members. Available online: https://www.edqm.eu/documents/52006/169295/Annex+3+to+the+GEON+Terms+of+Reference+List+of+GEON+Members+PA+PH+OMCL+%2809%29+45+R35.pdf/46a3fb83-972e-bc10-349a-ed76bd989080?t=1671089216501 (accessed on 10 April 2025).
- European Directorate for the Quality of Medicines & Healthcare, PA/PH OMCL (06) 81 R10 An “Aide-Memoire” for the Testing of Suspected Illegally Traded and Falsified Medicines. Available online: https://www.edqm.eu/en/testing-of-falsified-/-illegal-medicines (accessed on 10 April 2025).
- Koncz, D.; Tóth, B.; Roza, O.; Csupor, D. A Systematic Review of the European Rapid Alert System for Food and Feed: Tendencies in Illegal Food Supplements for Weight Loss. Front. Pharmacol. 2021, 11, 611361. [Google Scholar] [CrossRef]
- Wróbel, K.; Milewska, A.J.; Marczak, M.; Kozłowski, R. Dietary Supplements Questioned in the Polish Notification Procedure upon the Basis of Data from the National Register of Functional Foods and the European System of the RASFF. Int. J. Environ. Res. Public Health 2022, 19, 8161. [Google Scholar] [CrossRef]
- Vanhee, C.; Barhdadi, S.; Kamugisha, A.; Van Mulders, T.; Vanbrusselen, K.; Willocx, M.; Deconinck, E. The Development and validation of a Targeted LC-HRAM-MS/MS Methodology to Separate and Quantify p-Synephrine and m-Synephrine in Dietary Supplements and Herbal Preparations. Separations 2023, 10, 444. [Google Scholar] [CrossRef]
- Vanhee, C.; Jacobs, B.; Canfyn, M.; Malysheva, S.V.; Willocx, M.; Masquelier, J.; Van Hoorde, K. Quality Control and Safety Assessment of Online-Purchased Food Supplements Containing Red Yeast Rice (RYR). Foods 2024, 13, 1919. [Google Scholar] [CrossRef]
- Malysheva, S.V.; Guillaume, B.; Vanhee, C.; Masquelier, J. Determination of 16 Hydroxyanthracene Derivatives in Food Supplements Using LC-MS/MS: Method Development and Application. Toxins 2024, 16, 505. [Google Scholar] [CrossRef]
- Bundesinstitut fûr Risikobewertung (BfR), Melatoninhaltige Nahrungsergänzungsmittel: BfR Weist auf Mögliche Gesundheitsrisiken Hin (September 2024). Available online: https://www.bfr.bund.de/cm/343/melatoninhaltige-nahrungsergaenzungsmittel-bfr-weist-auf-moegliche-gesundheitsrisiken-hin-2024.pdf (accessed on 10 April 2025).
- Dutch National Institute for Public Health and the Environment (RIVM), Risk Assessment of Herbal Preparations Containing Huperzia serrata. Available online: https://rivm.openrepository.com/entities/publication/f69df6c1-f806-4b22-87d8-90ad5cfa69b6 (accessed on 10 April 2025).
- Dutch National Institute for Public Health and the Environment (RIVM), Risk Assessment of Herbal Preparations Containing Seed Extracts of Mucuna pruriens. Available online: https://rivm.openrepository.com/entities/publication/b196a66f-ff51-4679-bc74-d9b3929ca892 (accessed on 10 April 2025).
- Wang, R.; Yan, H.; Tang, X.C. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 2006, 27, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, C.; Liu, L.; Li, S. Protective effects of evodiamine in experimental paradigm of Alzheimer’s disease. Cogn. Neurodyn. 2018, 12, 303–313. [Google Scholar] [CrossRef]
- Lim, J.S.; Lee, J.; Kang, Y.; Park, H.T.; Kim, D.E.; Cha, J.K.; Park, T.H.; Heo, J.H.; Lee, K.B.; Park, J.M.; et al. Efficacy and safety of oxiracetam in patients with vascular cognitive impairment: A multicenter, randomized, double-blinded, placebo-controlled, phase IV clinical trial. Contemp. Clin. Trials 2023, 126, 107108. [Google Scholar] [CrossRef]
- Savchenko AIu Zakharova, N.S.; Stepanov, I.N. The phenotropil treatment of the consequences of brain organic lesions. Zhurnal Nevrol. I Psikhiatrii Im. S.S. Korsakova 2005, 105, 22–26. [Google Scholar]
- Birdsall, T.C. 5-Hydroxytryptophan: A clinically-effective serotonin precursor. Altern. Med. Rev. 1998, 3, 271–280. [Google Scholar] [PubMed]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Voznesenskaia, T.G.; Fokina, N.M.; Iakhno, N.N. Treatment of asthenic disorders in patients with psychoautonomic syndrome: Results of a multicenter study on efficacy and safety of ladasten. Zhurnal Nevrol. I Psikhiatrii Im. S.S. Korsakova 2010, 110 Pt 1, 17–26. [Google Scholar]
- Keller, S.; Polanski, W.H.; Enzensperger, C.; Reichmann, H.; Hermann, A.; Gille, G. 9-Methyl-β-carboline inhibits monoamine oxidase activity and stimulates the expression of neurotrophic factors by astrocytes. J. Neural. Transm. 2020, 127, 999–1012. [Google Scholar] [CrossRef]
- Buccafusco, J.J.; Weiser, T.; Winter, K.; Klinder, K.; Terry, A.V. The effects of IDRA 21, a positive modulator of the AMPA receptor, on delayed matching performance by young and aged rhesus monkeys. Neuropharmacology 2004, 46, 10–22. [Google Scholar] [CrossRef]
- Alkova, L.; Kozikowski, A.P.; Gale, K. The effects of huperzine A and IDRA 21 on visual recognition memory in young macaques. Neuropharmacology 2011, 60, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Wang, Y.; Du, Y.; Zeng, C.; Liu, Y.; Pan, H.; Ke, C. Current evidence for J147 as a potential therapeutic agent in nervous system disease: A narrative review. BMC Neurol. 2023, 23, 317. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Johe, K.; Hand, H.; Drouillard, A.; Russo, P.; Kay, G.; Kashambwa, R.; Hoeppner, B.; Flynn, M.; Yeung, A.; et al. A phase 2, double-blind, placebo-controlled study of NSI-189 phosphate, a neurogenic compound, among outpatients with major depressive disorder. Mol. Psychiatry 2020, 25, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Michelson, D.; Page, S.W.; Casey, R.; Trucksess, M.W.; A Love, L.; Milstien, S.; Wilson, C.; Massaquoi, S.G.; Crofford, L.J.; Hallett, M. An eosinophilia-myalgia syndrome related disorder associated with exposure to L-5-hydroxytryptophan. J. Rheumatol. 1994, 21, 2261–2265. [Google Scholar]
- Stone, C.J.; Lassen, K.; Abbott, J.; Hull, C. Eosinophilia-myalgia syndrome presenting with overlapping features of eosinophilic fasciitis and sarcoidal granulomas. JAAD Case Rep. 2024, 50, 115–118. [Google Scholar] [CrossRef]
- Cohen, P.A.; Avula, B.; Katragunta, K.; Khan, I. Recalls, Availability, and Content of Dietary Supplements Following FDA Warning Letters. JAMA 2022, 328, 393–395. [Google Scholar] [CrossRef]
- Sohutskay, D.O.; Suen, R.M.; Ali, F.; Rosenman, D.J. Dopamine Dysregulation Syndrome Presenting as Overuse of Mucuna pruriens Levodopa Supplement. J. Mov. Disord. 2024, 17, 357–359. [Google Scholar] [CrossRef]
- Gutierrez Higueras, T.; Calera Cortés, F.; Trives Muñoz, A.; Vicent Forés, S.; Sainz De La Cuesta Alonso, S. Attempted suicide by Melatonin overdose: Case report and literature review. Eur. Psychiatry 2022, 65 (Suppl. 1), S836–S837. [Google Scholar] [CrossRef]
- Lelak, K.; Vohra, V.; Neuman, M.I.; Toce, M.S.; Sethuraman, U. Pediatric Melatonin Ingestions—United States, 2012–2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 725–729. [Google Scholar] [CrossRef]
- Neuman, G.; Shehadeh, N.; Pillar, G. Unsuccessful suicide attempt of a 15 year old adolescent with ingestion of 5000 mg modafinil. J. Clin. Sleep. Med. 2009, 5, 372–373. [Google Scholar] [CrossRef]
- Man, K.K.C.; Coghill, D.; Chan, E.W.; Lau, W.C.Y.; Hollis, C.; Liddle, E.; Banaschewski, T.; McCarthy, S.; Neubert, A.; Sayal, K.; et al. Association of Risk of Suicide Attempts With Methylphenidate Treatment. JAMA Psychiatry 2017, 74, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Love, R.W.B. Aniracetam: An Evidence-Based Model for Preventing the Accumulation of Amyloid-β Plaques in Alzheimer’s Disease. J. Alzheimers Dis. 2024, 98, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.V.; Havens, J.R.; Walsh, S.L. Gabapentin misuse, abuse and diversion: A systematic review. Addiction 2016, 111, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Koturbash, I. Phenibut: A drug with one too many “buts”. Basic Clin. Pharmacol. Toxicol. 2024, 135, 409–416. [Google Scholar] [CrossRef]
- Xiao, S.-J.; Xu, X.-K.; Chen, W.; Xin, J.-Y.; Yuan, W.-L.; Zu, X.-P.; Shen, Y.-H. Traditional Chinese medicine Euodiae Fructus: Botany, traditional use, phytochemistry, pharmacology, toxicity and quality control. Nat. Prod. Bioprospect. 2023, 13, 6. [Google Scholar] [CrossRef]
- Solanki, R.; Patel, S. Evodiamine and its nano-based approaches for enhanced cancer therapy: Recent advances and challenges. J. Sci. Food Agric. 2024, 104, 8430–8444. [Google Scholar] [CrossRef]
- Working Group “Food Supplements” of Heads of Food Safety Agencies, First Report of the HoA Working Group “Food Supplements”. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/01_Lebensmittel/Internationales/report_HoA_WG_FS-en.html?nn=19745298 (accessed on 10 April 2025).
| Minimum (mg/Unit) | Maximum (mg/Unit) | Purity Raw Material % (w/w) | Therapeutic Range | |
|---|---|---|---|---|
| Melatonin | 0.5 | 20.3 | 55.1 | 1–10 mg/day |
| Modafinil | 88 | 197.3 | n.d. | 100–400 mg/day |
| Adrafinil a | 97 | 293 | n.d. | 300–600 mg/day |
| Methylphenidate | 25.5 | n.d. | 5 mg taken 2–3 times a day up to 60 mg/day | |
| L-Dopa b | 1.2 | 40 | n.d. | 50 mg several times a day, up to 1.6 g/day |
| Galantamine | 4.0 | n.d. | 4–12 mg twice a day | |
| Piracetam | 60 | 100 | n.d. | 1200–4800 mg/day |
| Aniracetam | 0.2 | 735 | n.d. | 200–750 mg/day |
| Gabapentin | 795 | n.d. | 600–3600 mg/day | |
| Meclofenoxate | 251.1 | n.d. | 50–400 mg/day | |
| Vinpocetin | 24 | n.d. | 5–20 mg taken 3 times daily | |
| Molecule | Minimum (mg/Unit) | Maximum (mg/Unit) | Purity, % (w/w) | Clinical Data or Usage Information |
| Noopept | 20 | 100 | Prescription medication, available in Russia, to treat traumatic brain injury, mood disorders, and cerebral vascular disease. The typical pharmacological dosage is 10 mg/day [7]. | |
| Oxiracetam | 270.7 | n.a. | A recent clinical trial with 800 mg oxiracetam twice daily for 36 weeks showed an increase in cognitive recovery after stroke [39]. | |
| Phenylpiracetam | n.a. | 100 | Prescription medication, available in Russia, promotes memory, increases concentration, is anti-depressant, anti-anxiety, and improves mood and physical performance. The typical pharmacological dosage consists of 100–200 mg/day [40]. | |
| 5-HTP | 3 | 100 | n.a. | 5-HTP may boost serotonin levels and have a positive effect on mood, depression, anxiety, sleep, appetite, and pain, although high-quality clinical studies are lacking. There have been reports associating eosinophilia myalgia syndrome with 5-HTP supplements that may have been contaminated. Dosages of 100–900 mg/day are described in clinical studies [41,42]. |
| Phenibut | 81 | 250 | 100 | Prescription medication, available in Russia, to treat anxiety, insomnia, and other issues. The typical pharmacological dosage is between 250 and 500 mg/day [7] |
| Bromantane | 33.2 | n.a. | Prescription medication, available in Russia, to treat “neurasthenia”. The typical pharmacological dosage is 50–100 mg/day [43]. | |
| 9- MBC | 11.8 | n.a. | No clinical data on humans were found. An in vitro study with astrocytes showed that this component could have neuroprotective and neuro-regenerative properties for dopaminergic neurons [44]. | |
| IDRA-21 | 12.1 | n.a. | No clinical data on humans were found. Studies in different animals (rats and monkeys) suggest improved memory after oral intake [45,46]. | |
| J-147 | 3.68 | n.a. | No clinical data on humans were found. Studies in different animals (rats and mice) suggest that oral intake improves memory and reduces anxiety [47]. | |
| NSI-189 | 19.1 | n.a. | This compound is currently being studied in clinical trials (phase 2) for its possible anti-depressant activity and pro-cognitive effects [48]. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanhee, C.; Deconinck, E.; George, M.; Hansen, A.; Hackl, A.; Wollein, U.; El-Atma, O.; Beerbaum, N.; Aureli, F.; Borioni, A.; et al. The Occurrence of Illicit Smart Drugs or Nootropics in Europe and Australia and Their Associated Dangers: Results from a Market Surveillance Study by 12 Official Medicines Control Laboratories. J. Xenobiot. 2025, 15, 88. https://doi.org/10.3390/jox15030088
Vanhee C, Deconinck E, George M, Hansen A, Hackl A, Wollein U, El-Atma O, Beerbaum N, Aureli F, Borioni A, et al. The Occurrence of Illicit Smart Drugs or Nootropics in Europe and Australia and Their Associated Dangers: Results from a Market Surveillance Study by 12 Official Medicines Control Laboratories. Journal of Xenobiotics. 2025; 15(3):88. https://doi.org/10.3390/jox15030088
Chicago/Turabian StyleVanhee, Celine, Eric Deconinck, Mark George, Andrew Hansen, Andreas Hackl, Uwe Wollein, Oliver El-Atma, Nico Beerbaum, Federica Aureli, Anna Borioni, and et al. 2025. "The Occurrence of Illicit Smart Drugs or Nootropics in Europe and Australia and Their Associated Dangers: Results from a Market Surveillance Study by 12 Official Medicines Control Laboratories" Journal of Xenobiotics 15, no. 3: 88. https://doi.org/10.3390/jox15030088
APA StyleVanhee, C., Deconinck, E., George, M., Hansen, A., Hackl, A., Wollein, U., El-Atma, O., Beerbaum, N., Aureli, F., Borioni, A., Poplawska, M., Blazewicz, A., Roschel, K., Marson, C., Mendoza Barrios, M., Hakkarainen, B., Blomgren, A., Bakker-‘t Hart, I., & Miquel, M. (2025). The Occurrence of Illicit Smart Drugs or Nootropics in Europe and Australia and Their Associated Dangers: Results from a Market Surveillance Study by 12 Official Medicines Control Laboratories. Journal of Xenobiotics, 15(3), 88. https://doi.org/10.3390/jox15030088








