Abstract
The present pilot study aimed to investigate whether common single nucleotide polymorphisms (SNPs) in the gene encoding glutathione S-transferase omega 1 (GSTO1), both individually and in combination with variants of the catalytic subunit of the glutamate cysteine ligase (GCLC) gene and environmental risk factors, are associated with the risk of psoriasis. The research included a total of 944 participants, comprising 474 individuals diagnosed with psoriasis and 470 healthy control subjects. Five common SNPs in the GSTO1 gene—specifically, rs11191736, rs34040810, rs2289964, rs11191979, and rs187304410—were genotyped in the study groups using the MassARRAY-4 system. The allele rs187304410-A (OR = 0.19, 95% CI 0.04–0.86, Pperm = 0.02) and the genotype rs187304410-G/A (OR = 0.19, 95% CI 0.04–0.85, Pperm = 0.01) were found to be associated with psoriasis in females. The model-based multifactor dimensionality reduction approach facilitated the identification of higher-order epistatic interactions between the variants of the GSTO1 and GCLC genes (Pperm < 0.0001). These interactions, along with the risk factor of alcohol abuse, collectively contribute to the pathogenesis of psoriasis. This study is the first to demonstrate that polymorphisms in the GSTO1 gene, both individually and in combination with variants of the GCLC gene and alcohol abuse, are associated with an increased risk of psoriasis.
1. Introduction
Psoriasis is a chronic dermatosis characterized by immune-mediated inflammation, resulting in the formation of thickened, scaly erythematous plaques [1]. The global prevalence of psoriasis is approximately 2%, with psoriasis vulgaris, also known as plaque-type psoriasis, representing the most common subtype and accounting for around 90% of all cases [2,3]. Psoriasis is commonly understood as a complex multifactorial disorder resulting from the interplay between genetic and environmental factors [4]. In recent decades, substantial progress has been achieved in elucidating the genetic mechanisms underlying psoriasis, primarily facilitated by genome-wide association studies that have identified more than sixty regions associated with disease susceptibility [4]. These investigations have highlighted the pathogenic significance of genes involved in the activation of Th17 cells and have also uncovered rare genetic variants associated with distinct forms of psoriasis [4,5]. Numerous extrinsic factors—such as mechanical stress, air pollutants, sun exposure, medications, vaccinations, infections, and lifestyle choices—and intrinsic factors—including obesity, diabetes mellitus, dyslipidemia, hypertension, and mental stress—have been identified as triggers and exacerbating factors for psoriasis [6]. Research conducted on extensive twin cohorts indicates that genetic factors account for 60–75% of the variability in susceptibility to psoriasis, while the remaining variation is attributed to non-shared environmental influences [7]. Despite the well-established role of environmental factors in the pathogenesis of psoriasis [8], exploring the mechanisms by which these factors disrupt the body’s equilibrium and contribute to the onset and progression of the disease remains a significant challenge.
In recent years, a growing body of research has emerged that underscores the significant role of chemical exposure, particularly air pollutants, in the etiology of psoriasis. Numerous air pollutants, including polycyclic aromatic hydrocarbons, volatile organic compounds, oxides, particulate matter, ozone, heavy metals, and ultraviolet radiation, have been shown to adversely affect the skin and increase the risk of psoriasis [9,10]. A recent large prospective cohort study involving 474,055 participants demonstrated that long-term exposure to air pollution—measured in terms of nitrogen dioxide, nitrogen oxides, fine particulate matter with a diameter of less than 2.5 µm, and particulate matter with a diameter of less than 10 µm—is associated with an increased risk of psoriasis [11]. Additionally, this study found an interaction between air pollution and genetic susceptibility that jointly contributes to the risk of psoriasis [11]. Furthermore, environmental exposure to toxic metals such as barium, cesium, antimony, uranium, lead, mercury, and cadmium has been found to compromise immunity and increase inflammation, thereby predisposing individuals to the development and exacerbation of psoriasis [12,13,14,15,16].
In order to mitigate the harmful effects of chemical pollutants, the skin expresses enzymes that are involved in xenobiotic biotransformation, with glutathione S-transferases (GSTs) playing a particularly significant role [17,18]. The primary biological function of glutathione S-transferases is to facilitate the detoxification of chemicals by catalyzing their conjugation with reduced glutathione [19]. Numerous studies have demonstrated an increased expression of glutathione S-transferases in affected skin regions compared to unaffected tissues in patients with psoriasis [20,21,22]. This observation suggests, first, the critical involvement of these enzymes in the pathogenesis of the disease, and second, that the elevated levels of GSTs in the affected skin may serve a protective function by neutralizing toxic substances that accumulate in the dermal layers. Since the genetic variability of glutathione S-transferases can influence the activity and expression of these enzymes, thereby determining individual characteristics related to skin protection against chemical exposures, polymorphisms in the GST genes have become a focal point for genetic studies on psoriasis in recent years. The vast majority of studies on psoriasis have concentrated on the deletion polymorphisms of the glutathione S-transferase genes GSTM1 and GSTT1 [23,24,25,26,27,28,29,30,31]. These studies have demonstrated associations with susceptibility to psoriasis, its clinical features, and the efficacy of various therapeutic approaches for the condition. However, no studies to date have been conducted to investigate whether polymorphisms in the gene encoding glutathione S-transferase omega 1 (GSTO1) contribute to susceptibility to psoriasis. Therefore, the present pilot study aimed to determine whether common single nucleotide polymorphisms (SNPs) in the GSTO1 gene are associated with the risk of psoriasis.
The GSTO1 gene was selected for this study due to its high expression in skin cells (data from https://www.gtexportal.org, accessed on 10 March 2025), where it is likely to play a crucial role in the biotransformation of xenobiotics that penetrate the skin from the environment or enter the bloodstream. An additional objective was to investigate whether polymorphisms in the GSTO1 gene, in conjunction with variants of the catalytic subunit of the glutamate cysteine ligase (GCLC) gene—which encodes the first enzyme in the cellular glutathione (GSH) biosynthetic pathway—contribute to a polygenic predisposition to psoriasis. Thus, the selection of genes is justified by their functionality and potential collaborative roles in the pathogenesis of psoriasis, particularly regarding their involvement in redox homeostasis and xenobiotic metabolism.
The present study found that polymorphisms in the GSTO1 gene contribute to susceptibility to psoriasis, with differing effects observed between males and females. In particular, the allele rs187304410-A and the genotypes rs187304410-G/A and rs187304410-A/A were found to be protective against the risk of psoriasis in females. Conversely, the allele rs11191736-T was associated with an increased risk of the disease in males. Moreover, GSTO1 polymorphisms exhibited synergistic effects on disease risk in both males and females. In addition, joint effects on disease risk were identified for polymorphisms in the GSTO1 and GCLC genes. Environmental risk factors, such as alcohol abuse and cigarette smoking, also exhibited joint effects with GSTO1 and GCLC polymorphisms on the risk of developing psoriasis. The present study is the first to demonstrate that polymorphisms in the GSTO1 gene, both individually and in combination with GCLC polymorphisms and glutathione-depleting environmental risk factors, contribute to susceptibility to psoriasis. This study highlights the critical role of compromised glutathione detoxification mechanisms in the development of the disease.
2. Materials and Methods
2.1. Study Patients
All phases of data collection and analysis were conducted in accordance with the principles outlined in the Declaration of Helsinki. Informed written consent was obtained from human participants in compliance with protocols approved by the Ethics Review Committee of Kursk State Medical University. The study was conducted in a case–control design and involved a cohort of 944 unrelated individuals of European ancestry, primarily consisting of Russians from the Kursk region. This group included 474 patients diagnosed with psoriasis and 470 healthy controls. Patient enrollment for the study involving individuals with psoriasis occurred at Medvenka Central District Hospital in the Kursk region, the Center for Medical Examinations and Prevention in Kursk, and the Kursk Regional Multidisciplinary Clinical Hospital, covering the period from September 2018 to December 2021. The control group, comprising individuals without chronic diseases, was drawn from our previous research efforts [32,33,34]. The mean age of patients with psoriasis was 44.3 ± 13.6 years, while the mean age of individuals in the control group was 55.3 ± 6.7 years. The patients with psoriasis (252 males and 222 females) and the control group (234 males and 236 females) were matched by gender.
2.2. The Inclusion/Exclusion Criteria in the Study Groups and Diagnosis of Psoriasis
The inclusion criteria for the psoriasis group were as follows: (1) a diagnosis of psoriasis confirmed by certified dermatologists through the assessment of the characteristic clinical presentation of skin lesions and their specific locations [35]; (2) Slavic ethnicity; (3) age 18 years or older; and (4) consent to participate in the study. The exclusion criteria were as follows: (1) the presence of chronic infectious diseases such as HIV or hepatitis or severe chronic conditions that could predispose individuals to the onset of psoriasis; (2) non-Slavic ethnicity; (3) current pregnancy; (4) individuals undergoing biologic therapy at the time of recruitment; and (5) refusal to participate in the study. The case group comprised individuals diagnosed with various forms of psoriasis, including classic plaque psoriasis, palmoplantar psoriasis, seborrheic psoriasis, scalp psoriasis, the von Zumbusch variant of generalized pustular psoriasis, inverse psoriasis, guttate psoriasis, and erythrodermic psoriasis. Additionally, the group included patients with comorbid conditions associated with psoriasis, such as psoriatic arthritis and onychodystrophy. The Psoriasis Area and Severity Index (PASI) was utilized as a clinical tool for assessing the severity of psoriasis progression. The criteria for inclusion in the control group were established as follows: (1) absence of chronic illnesses; (2) identification as belonging to a Slavic ethnicity; (3) age of 18 years or older; and (4) provision of informed consent to participate in the study. Conversely, the exclusion criteria included (1) identification as a non-Slavic ethnicity; and (2) refusal to provide consent for participation in the study.
2.3. Interviewing of Patients
Participants in the study completed a validated questionnaire administered by a physician [36] to evaluate risk factors associated with psoriasis, including cigarette smoking, alcohol consumption, and decreased consumption of fresh fruits and vegetables [37]. Interest in these risk factors arises from their harmful impact on the levels of endogenous glutathione, a vital molecule employed by glutathione S-transferases in the detoxification of xenobiotics [38]. The alcohol consumption, cigarette smoking, and dietary habits of the study participants were evaluated as previously described [39,40]. Data regarding smoking status (ever/never) were collected from all participants diagnosed with psoriasis, as well as from healthy controls. Information pertaining to alcohol consumption was obtained from all patients with psoriasis and a subset of 220 individuals from the control group. Alcohol consumption patterns were evaluated based on the number of alcoholic beverages consumed weekly, as previously outlined in the literature [38,39]. In summary, participants were classified into two categories based on their reported frequency of alcohol intake: (1) individuals who consumed alcohol 1 to 2 days per month or less, and (2) those who consumed alcohol on 1 or more days per week. The latter group was identified as alcohol abusers.
2.4. SNP Selection
Single nucleotide polymorphisms of the GSTO1 gene were selected for this study based on the following criteria: (1) a minor allele frequency of ≥1% in the European population (Ensembl data, www.ensembl.org, accessed on 4 February 2023); (2) the presence of an SNP with GSTO1 expression quantitative trait loci (eQTL) and/or splicing quantitative trait loci (sQTL) in the skin, specifically in sun-exposed areas (lower leg) and non-sun-exposed areas (suprapubic), according to data from the GTEx portal (https://gtexportal.org, accessed on 16 December 2024); (3) linkage disequilibrium (r2 < 0.8) among the SNPs of interest (HapMap data for the European population); and (4) the feasibility of joint SNP detection in a multiplex panel using the MALDI-TOF mass spectrometry method. As a result, five common SNPs of the GSTO1 gene were selected for genotyping: rs11191736, rs34040810, rs2289964, rs11191979 (tagSNP), and rs187304410.
2.5. Genetic Analysis
All genetic investigations were performed at the Research Institute for Genetic and Molecular Epidemiology, situated at Kursk State Medical University in Kursk, Russia. Venous blood samples were obtained from the cubital vein of the study participants and collected in EDTA-coated tubes. These samples were promptly frozen and stored at −20 °C until they were processed. Genomic DNA was isolated utilizing the conventional methods of phenol/chloroform extraction followed by ethanol precipitation. Genotyping of the selected polymorphisms was conducted using the MALDI-TOF mass spectrometry iPLEX platform on the MassArray-4 system (Agena Bioscience, Inc., San Diego, CA, USA). The methodologies for sample preparation and single nucleotide polymorphism (SNP) genotyping using the MassArray-4 system are thoroughly detailed in our latest publication [41]. The primer sequences utilized for genotyping can be provided upon request. In order to ensure quality control, 5% of the DNA samples were subjected to duplicate genotyping, with the researchers remaining unaware of the case–control status. The concordance rate of the control genotyping was greater than 99%.
2.6. Statistical and Bioinformatics Analysis
The estimation of statistical power for the association analysis was conducted using the GAS power calculator, as documented in previous research [39]. In particular, it was estimated that the overall analysis, which included 474 cases and 470 controls, would enable the detection of a genotype relative risk (GRR) ranging from 1.30 to 1.45, with a statistical power of 82% to 98%. Furthermore, in the analysis of groups stratified by sex and risk factors, a GRR of 1.40 to 1.50 could be detected with a power of 76% to 83%, utilizing a significance level of α = 0.05. Fisher’s exact test was employed to evaluate the distribution of genotype frequencies in relation to the Hardy–Weinberg equilibrium (HWE). The frequencies of alleles and genotypes within the study cohorts, along with their correlations to the risk of developing psoriasis, were analyzed using PLINK software version 1.9 [42]. Logistic regression analysis was employed to investigate the relationships between polymorphisms in the GSTO1 gene and the risk of psoriasis. The unadjusted odds ratios (OR) and 95% confidence intervals (95% CI) were calculated to assess the associations between single nucleotide polymorphisms (SNPs) and the corresponding phenotypes. In the context of SNP–disease associations, various genetic models—including allelic, recessive, dominant, and log-additive models—were assessed. Genetic models reflect how alleles at a specific single nucleotide polymorphism interact to influence disease phenotypes. In particular, the allelic model implies a comparison of allele frequencies between cases and controls. Chi-square tests were conducted utilizing a 2 × 2 contingency table to assess both dominant and recessive genetic models. For the log-additive model, Cochran–Armitage trend tests were employed with a 2 × 3 contingency table. A model with the lowest p-value was selected for interpreting the SNP–disease association. The replication of associations between polymorphisms in the GSTO1 gene and psoriasis was conducted using the Gene ATLAS database from the UK Biobank (http://geneatlas.roslin.ed.ac.uk, accessed on 10 September 2024). Additionally, haplotype analysis of the GSTO1 gene was conducted using Haploview software, version 4.2. p-Values (Pperm) for associations involving alleles, genotypes, and haplotypes were calculated using adaptive permutation methods with PLINK and Haploview software. The relationships between pairwise genotype combinations (diplotypes) and the risk of developing psoriasis were evaluated using the chi-squared test using the STATISTICA 13.0 software. Gene–gene (G × G or SNP–SNP) and gene–environment (G × E) interactions were analyzed using a model-based multifactor dimensionality reduction method (mbmdr) [43]. This non-parametric, model-free bioinformatics approach enables the identification of non-additive interactions among discrete variables, such as SNPs and risk factors that influence the development of psoriasis. The mbmdr methodology facilitates the simultaneous evaluation of multiple variables, including gene polymorphisms and environmental factors, by reducing the dimensionality of the calculated parameters [43]. By detecting complex interactions between environmental and genetic factors contributing to disease risk, this method can reveal relationships that conventional parametric statistics may overlook [43]. Among the environmental risk factors contributing to psoriasis, particular attention has been directed toward those that adversely affect endogenous levels of glutathione. These factors include excessive alcohol consumption, tobacco use, and a dietary deficiency of fresh fruits and vegetables [39]. Regarding the risk of psoriasis, we evaluated second-, third-, and fourth-order G × G and G × E models that included the GSTO1 and GCLC gene polymorphisms, along with the mentioned risk factors. The mbmdr software version 3.5.3 enables the comparison of high- and low-risk mbmdr models between case and control groups, thereby identifying the optimal n-th-order G × G and G × E interactions with the lowest Pperm values that contribute to the risk of psoriasis. The overall impact of each polymorphism in the studied genes, along with environmental factors, on the polygenic predisposition to psoriasis was evaluated by aggregating all mbmdr models that included a specific genetic variant or risk factor.
3. Results
3.1. Association Between GSTO1 Gene Polymorphisms and the Risk of Psoriasis
The genotype frequencies for all single nucleotide polymorphisms of GSTO1 were found to be in HWE in both the case and control groups (p > 0.05). We conducted an analysis of the relationships between polymorphisms in the GSTO1 gene and the risk of developing psoriasis, examining both the overall population and subgroups stratified by sex. A summary of the associations between GSTO1 gene polymorphisms and psoriasis risk for both the entire cohort and the sex-stratified groups is presented in Table 1. No statistically significant associations were found between SNPs of the GSTO1 gene and the risk of developing psoriasis when comparing the overall case and control groups. The sex-stratified analysis presented in Table 1 indicates that two specific polymorphisms are significantly associated with the risk of psoriasis: rs11191736 (allelic model) in males and rs187304410 (dominant model) in females.

Table 1.
A summary of associations between GSTO1 gene polymorphisms and psoriasis risk in the entire group and sex-stratified groups.
Table 2 presents the genotype and allele frequencies of the GSTO1 gene, along with their associations with the risk of psoriasis, analyzed for both the entire group and stratified by sex. In the male groups, allele rs11191736-T was found only among patients with psoriasis (Pperm = 0.017). Allele rs187304410-A (OR = 0.19, 95% CI 0.04–0.86, Pperm = 0.02) and genotype rs187304410-G/A (OR = 0.19, 95% CI 0.04–0.85, Pperm = 0.01) were found to confer protection against the risk of psoriasis in females.

Table 2.
Genotype and allele frequencies of the GSTO1 gene and their associations with psoriasis risk in the entire group and sex-stratified groups.
Subsequently, we conducted a replication analysis to investigate the associations between polymorphisms of the GSTO1 gene and susceptibility to psoriasis in the large cohort from the UK Biobank. None of the polymorphisms we studied demonstrated a statistically significant association with the risk of developing psoriasis in this cohort.
The difficulty in replicating associations between SNPs and diseases may be partially attributed to genetic variations across different populations, as we recently demonstrated [44,45]. This observation prompted us to conduct an association analysis of psoriasis in relation to all SNPs of the GSTO1 gene that were genotyped within the UK Biobank cohort. As a result, 40 SNPs of the GSTO1 gene within the UK Biobank cohort were identified as being associated with an increased risk of psoriasis, with a p-value of ≤0.05, as presented in Supplementary Table S1.
3.2. The Combined Impact of GSTO1 Gene Polymorphisms on Psoriasis Risk
The combined influence of polymorphisms in the GSTO1 gene on the risk of developing psoriasis was assessed using haplotype and diplotype analyses. Table 3 presents the GSTO1 haplotypes and their correlation with the risk of psoriasis, analyzed for both the overall population and stratified by sex. This study identified four prevalent haplotypes of GSTO1, designated as H1–H4, which exhibited a frequency exceeding 2% within the study populations. As shown in Table 3, none of the GSTO1 haplotypes were found to be associated with the risk of psoriasis (Pperm > 0.05). SNP rs11191736 exhibited a positive linkage disequilibrium (p < 0.001) with the polymorphisms rs34040810 (D’ = 0.497), rs2289964 (D’ = 0.441), and rs187304410 (D’ = 0.426). Polymorphism rs34040810 was associated with rs2289964 (D’ = 0.992) and rs187304410 (D’ = 0.605). Additionally, rs187304410 showed a correlation with rs2289964 (D’ = 0.358).

Table 3.
Haplotypes of the GSTO1 gene and their association with psoriasis risk in the entire group and sex-stratified groups.
Supplementary Table S2 presents the genotype combinations (diplotypes) of GSTO1 and their associations with the risk of psoriasis. Two diplotypes, rs11191736C/C × rs34040810C/C (OR = 0.21, 95% CI 0.04–0.95, p = 0.03) and rs2289964C/T × rs11191979T/T (OR = 2.50, 95% CI 1.06–5.92, p = 0.05), along with three diplotypes—rs11191736C/C × rs187304410G/G (OR = 5.99, 95% CI 1.34–26.89, p = 0.01), rs34040810C/C × rs187304410G/G (OR = 3.91, 95% CI 1.09–14.05, p = 0.02), and rs34040810C/C × rs187304410G/A (OR = 0.24, 95% CI 0.06–0.98, p = 0.05)—were found to be associated with the risk of psoriasis risk in males and females, respectively. Nevertheless, none of these associations remained significant after applying the Benjamini–Hochberg procedure for multiple testing corrections.
Considering that glutathione S-transferases utilize glutathione for the detoxification of xenobiotics, it is essential to investigate the potential interactive effects of polymorphisms in the GSTO1 gene and the gene encoding the catalytic subunit of glutamate cysteine ligase (GCLC) on susceptibility to psoriasis. Genotyping data for six common polymorphisms of the GCLC gene in patients with psoriasis and controls were obtained from our previous study [39]. As shown in Supplementary Table S3, diplotypes GSTO1 rs34040810C/C × GCLC rs648595G/G (OR = 0.70 95% CI 0.50–0.99, p = 0.04), GSTO1 rs11191979T/T × GCLC rs542914C/C (OR = 1.44 1.02–2.02, p = 0.04), GSTO1 rs11191979T/C × GCLC rs542914C/C (OR = 0.69 95% CI 0.48–0.99, p = 0.04), and GSTO1 rs11191979T/C × GCLC rs648595G/G (OR = 0.55 95% CI 0.33–0.94, p = 0.03) were associated with the risk of psoriasis. These associations also did not survive after adjustment for multiple testing.
3.3. The Role of Gene–Gene and Gene–Environment Interactions in the Risk of Psoriasis
The comprehensive assessment of gene-by-gene interactions poses a challenge commonly known as the “curse of dimensionality”. This phenomenon arises in the context of epistatic analysis (epistasis is a genetic phenomenon whereby the impact of a mutation in a particular gene is influenced by the presence or absence of mutations in one or more additional genes), where the number of SNPs increases exponentially, significantly limiting the effectiveness of traditional parametric statistical methods [46,47,48]. In order to address this challenge, we employed the model-based multifactor dimensionality reduction approach, as outlined by Calle and colleagues [43], to assess the interactions between genes and between genes and environmental factors that are associated with susceptibility to psoriasis.
The distribution of environmental risk factors assessed in the study participants is presented in Table 4. The prevalence of alcohol abuse was higher among patients with psoriasis compared to healthy controls, both in the overall group and when stratified by sex (p < 0.0001). In addition, decreased consumption of fresh fruits and vegetables was associated with an increased risk of psoriasis in females (p = 7.1 × 10−8) but not in males (p = 0.07). No differences in cigarette smoking were observed between the case and control groups.

Table 4.
Environmental risk factors among study participants.
Second-, third-, and fourth-order G × G and G × E models, which include combinations of SNPs at the GSTO1 and GCLC genes, along with the aforementioned risk factors, were analyzed to identify associations with the risk of psoriasis. Table 5 summarizes the number of mbmdr models that demonstrate significant associations between SNP × risk factor interactions and the risk of developing psoriasis. A total of 12 second-order, 68 third-order, and 239 fourth-order statistically significant (Pperm < 0.05) mbmdr models associated with the risk of psoriasis were established for the entire group. The primary findings indicated that the vast majority of models linking the risk of psoriasis were established due to gene–environment interactions, with alcohol abuse emerging as a significant risk factor. Notably, the highest number of gene–environment interactions was identified between alcohol abuse and the polymorphisms of the GSTO1 and GCLC genes. Furthermore, many complex (third- and fourth-order) mbmdr models comprised cigarette smoking along with the interactions of gene polymorphisms and alcohol abuse.

Table 5.
Summary of the number of mbmdr models of SNP × risk factor interactions significantly associated with psoriasis risk.
Distinct characteristics of gene–environment interactions in males and females were identified. Notably, among the top five n-th-order mbmdr models, the risk factor of smoking was associated with 11 G × E interactions in females, while only one gene–smoking interaction (ALCOHOL × SMOKE × GCLC rs542914 × GSTO1 rs11191979) was identified in males. As illustrated in Table 5, statistically significant differences between sexes were observed in both third-order and fourth-order models. In particular, the number of models involving ALCOHOL × GCLC (1 SNP) × GSTO1 (1 SNP) was twice as high in males compared to females. The ALCOHOL × GSTO1 (2 SNPs) × GCLC (1 SNP) model accounted for nearly 36% of the fourth-order models, with no corresponding model identified in females. In contrast, the number of ALCOHOL × SMOKE × GCLC (1 SNP) × GSTO1 (1 SNP) and ALCOHOL × SMOKE × GSTO1 (2 SNPs) mbmdr models was higher in females than in males. The top five n-th-order mbmdr G×E models associated with the risk of psoriasis in both males and females are presented in Table 6 and Table 7, respectively. Polymorphisms of the GSTO1 gene were identified in 19 of the best mbmdr models for males and 14 models for females. Notably, the second-order mbmdr models for females are exclusively composed of polymorphisms in the GSTO1 gene. In contrast, the models for males are influenced by both gene–environment interactions, incorporating three SNPs of GSTO1 and two variants of GCLC. Additionally, SNPs of the GCLC gene were detected in nine models for males and five models for females.

Table 6.
The best n-th-order mbmdr models of SNP × risk factor interactions significantly associated with psoriasis risk in males.

Table 7.
The best n-th-order mbmdr models of SNP × risk factor interactions significantly associated with psoriasis risk in females.
4. Discussion
Glutathione S-transferases (GSTs) play a crucial role in detoxifying harmful xenobiotics, inactivating endogenous compounds resulting from oxidative stress, and synthesizing essential biological molecules such as leukotrienes, prostaglandins, testosterone, and progesterone [19]. In the context of xenobiotic biotransformation, GSTs utilize the antioxidant glutathione to facilitate the detoxification of both exogenous and endogenous compounds [19,38]. GSTs also exhibit a degree of antioxidant activity, particularly in relation to the inactivation of end products resulting from lipid peroxidation [49,50]. Hence, the association of these enzymes with oxidative stress can be attributed to the depletion of glutathione levels, which are utilized for neutralizing foreign chemical compounds. The omega class glutathione S-transferases (GSTO) are cytosolic enzymes that have been identified in various species, including humans [51]. In contrast to other classes of GSTs, which typically contain tyrosine or serine residues in their active sites, the active sites of GSTO enzymes feature an N-terminal cysteine residue that can bind to glutathione [52]. There is a growing body of evidence indicating that GSTO enzymes play a significant role in the detoxification of various exogenous stressors. In vitro studies indicate that human GSTO1 modulates the ryanodine receptor in the sarcoplasmic/endoplasmic reticulum, which is essential for Ca2+ release during excitation–contraction coupling in cardiac and skeletal muscles [53].
Furthermore, these studies indicated that human GSTO1 modulates the signaling pathway in c-Jun N-terminal kinase (JNK)-mediated apoptosis and activates interleukin-1β, a crucial mediator of inflammation [51]. Human GSTO1 has also been shown to regulate lipopolysaccharide-induced inflammatory responses in macrophages [54]. GSTO1 plays a critical role in redox homeostasis by influencing the glutathionylation and deglutathionylation of target proteins [51].
Oxidative stress is vital in psoriasis pathogenesis, prompting studies on the link between functionally significant glutathione S-transferase gene polymorphisms and disease risk. Certain studies indicate significant correlations between these variants and an increased susceptibility to psoriasis; however, other research has not found any associations with the risk of the disease [55,56]. The present study is the first to investigate whether polymorphisms in the GSTO1 gene contribute to the risk of developing psoriasis. No statistically significant correlations were found between polymorphisms of the GSTO1 gene and psoriasis risk when analyzing the overall case and control groups. The sex-stratified analysis revealed that two polymorphisms—specifically, rs11191736 in males and rs187304410 in females—are associated with the risk of psoriasis. Although the associations of psoriasis with the rs11191736 and rs187304410 polymorphisms could not be replicated in the overall population of the UK Biobank, 40 other SNPs in the GSTO1 gene have been identified as nominally associated with the risk of psoriasis (Supplementary Table S1). These findings clearly highlight the significant role of GSTO1 gene polymorphisms in psoriasis susceptibility. Analyses of haplotypes and diplotypes indicated that there were either no significant joint effects or only weak associations between the combinations of GSTO1 genotypes and the risk of developing psoriasis. Nonetheless, bioinformatics modeling of the non-linear interactions between GSTO1 and GCLC gene polymorphisms using the mbmdr method has revealed the existence of higher-order epistatic interactions between these genes, as well as with environmental risk factors, in relation to the risk of developing psoriasis. Moreover, the vast majority of mbmdr G x E models incorporated the combined effects of alcohol abuse and the polymorphisms of the GSTO1 and GCLC genes on the risk of developing the disease. Research indicates that alcohol abuse significantly affects both the onset and progression of psoriasis [57,58]. Our recent study identified the joint effects of alcohol consumption and GCLC gene polymorphisms on psoriasis risk [39]. Apparently, these gene–environment interactions can be elucidated by the fact that, on one hand, the consumption of substantial amounts of alcohol is linked to the production of a significant number of reactive oxygen species and oxidative stress [59,60]. This process depletes the reserves of reduced glutathione and causes damage to liver tissue, where the majority of the body’s glutathione is synthesized [61,62]. On the other hand, the development of oxidative stress may be exacerbated by reduced glutathione synthesis resulting from decreased expression of the GCLC gene and/or increased utilization of reduced glutathione by the GSTO1 enzyme.
To understand the mechanisms by which polymorphisms are associated with the onset of psoriasis, it is crucial to analyze the functional annotation data of the GSTO1 polymorphisms. SNP rs187304410, which has been associated with a reduced risk of psoriasis in females according to the GTEx portal (https://www.gtexportal.org/home/, accessed on 8 December 2024), correlates with alternative splicing events of the GSTO1 and GSTO2 genes in suprapubic and lower leg skin, as well as in cultured skin fibroblasts (https://www.gtexportal.org/home/, accessed on 8 December 2024). One of the alternative splicing sites is situated within intron 9 and is associated with a reduction in the expression of one GSTO1 isoform. Conversely, the other site, located in intron 7, is linked to an elevated production of a different isoform of the enzyme. It is well-established that alternative splicing can lead to the generation of multiple isoforms, some of which remain unknown [63]. To address the question regarding the properties and functions of the presumed GSTO1 isoforms with altered functional activity, it is essential to conduct experimental studies aimed at determining enzyme activity, substrate specificity, and other characteristics. In the blood, the polymorphism rs187304410 (allele A) is strongly associated with the expression levels of GSTO2, according to data from the eQTLGen consortium (https://www.eqtlgen.org/cis-eqtls.html, accessed on 8 December 2024). Other SNPs were found to be correlated with the expression levels of the GSTO1 and GSTO2 genes in the skin. The GSTO1 and GSTO2 genes are situated in proximity to one another on the same chromosomal segment, 10q25.1. Their transcriptional activity in the skin, specifically in foreskin fibroblast primary cells, seems to be regulated by shared enhancers found in the adjacent genomic region 104470800-104472000, as indicated by the Super-Enhancer database (http://www.licpathway.net/sedb/, accessed on 8 December 2024).
As mentioned above, GSTOs are involved in the biotransformation of xenobiotics and play a crucial role in protecting the skin from damage caused by environmental chemicals that penetrate both the superficial and deeper layers of the skin through transcutaneous and systemic routes [10,64]. Hence, the skin is one of the primary targets of environmental pollutants, and the disruption of the skin barrier due to inadequate xenobiotic biotransformation may potentially cause or exacerbate psoriasis. In particular, certain chemicals, especially drugs such as hydroxychloroquine [65], imiquimod [66], and lithium [67], have been identified as agents that can induce and exacerbate psoriasis. Per- and polyfluoroalkyl substances (PFAS), a class of synthetic chemicals extensively used in various consumer products, have been found to contribute to the risk of psoriasis [68]. Human exposure to PFAS primarily occurs through ingestion and inhalation, with the main sources being food, drinking water, and airborne dust. GSTO1 and GSTO2 are involved in the biotransformation of inorganic arsenic and play a crucial role in the reduction of monomethylarsonic acid, a highly toxic intermediate in the methylation process of inorganic arsenic [69,70]. Monomethylarsonic acid, a metabolite of arsenic, is the active ingredient in over 600 herbicides used in various agricultural products, including cotton, almonds, and oranges, as well as in landscaping applications [71]. GSTO2, like GSTO1, exhibits glutathione-dependent thiol transferase activity and shows significantly higher dehydroascorbate reductase activity—approximately 70 to 100 times greater than that of GSTO1 [70]. This enhanced activity may contribute to the regeneration of ascorbic acid, thereby providing protection against oxidative stress. This function of GSTO2 aligns with research findings indicating that ascorbic acid alleviates symptoms associated with skin lesions in psoriasis [37,72].
The present study revealed an intriguing finding regarding sexual dimorphism in the associations between psoriasis and single nucleotide polymorphisms in the GSTO1 gene, both individually and in combination with GCLC variants and environmental risk factors. In females, the impact of polymorphisms in the GSTO1 gene on the risk of psoriasis was more pronounced than that of the GCLC gene. In contrast, in males, the polymorphisms of both genes contributed nearly equally to the risk of developing the disease. These findings are not surprising, as genetic markers often demonstrate sexual dimorphism in their associations with various multifactorial diseases [73,74], including psoriasis [75,76]. The fundamental biological mechanisms that contribute to these sex differences are not yet fully elucidated. However, we believe that the sexual dimorphism observed in the associations between psoriasis and the SNPs of the GSTO1 and GCLC genes—both individually and in interaction with one another and environmental factors—can be attributed to several reasons. In our view, the most plausible explanation for the observed differences in SNP–disease associations between men and women is the influence of environmental risk factors. Specifically, alcohol abuse and a dietary deficiency in fresh fruits and vegetables have been linked to psoriasis, as supported by our data (Table 4) and corroborated by existing literature [57,58,59,77,78]. It is well established that the identified risk factors are associated with a reduction in endogenous glutathione. This reduction may occur due to its depletion from the toxic effects of ethanol [79] or insufficient synthesis resulting from a decreased intake of amino acid precursors of glutathione, which are found in high concentrations in unprocessed, plant-based foods [80]. The effects of functionally inferior alleles of the polymorphisms in the studied genes appear to exacerbate the deficiency of glutathione in psoriasis. This deficiency arises from insufficient synthesis involving glutamate cysteine ligase and/or excessive utilization by GSTO1 for the detoxification of xenobiotics. Thus, the unidirectional influence of environmental factors, combined with polymorphisms in GSTO1 and GCLC, seems to disrupt glutathione metabolism. This disruption may lead to conditions that promote oxidative stress and contribute to the onset of psoriasis. Another possible explanation for the sexual dimorphism observed in the associations between GSTO1 and GCLC polymorphisms and psoriasis is that women generally exhibit greater sensitivity to the toxic effects of chemical pollutants compared to men, as evidenced by some studies [15,81]. It is noteworthy that this phenomenon also appears to manifest at the level of associations between polymorphisms of genes encoding xenobiotic biotransformation enzymes, one of which is glutathione S-transferase omega 1. In other words, the genes we studied that encode biotransformation enzymes, similar to other genes involved in xenobiotic metabolism, may be differentially regulated in men and women, a fact that has been well established [82,83].
Our study has both strengths and limitations. This investigation is the first to explore the associations between GSTO1 gene polymorphisms and the risk of psoriasis. It is also one of the largest studies conducted on GST gene polymorphisms and their susceptibility to psoriasis. The application of the mbmdr methods enabled the identification of epistatic interactions between the polymorphisms of the GSTO1 gene and variants of GCLC in determining the risk of psoriasis. Given that our research is a pilot study, it is essential to conduct further investigations in independent populations to validate the associations identified between GSTO1 gene polymorphisms and the risk of psoriasis. Furthermore, as our study examined a relatively limited number of single nucleotide polymorphisms, it is advisable for future genetic association studies to explore the relationship between psoriasis and a broader array of polymorphisms, including those that have demonstrated associations with psoriasis in the UK Biobank cohorts (as detailed in the Supplementary Table S1). Additionally, since the polymorphisms analyzed in the GSTO1 gene are located in noncoding regions, any phenotypic implications should be approached with caution, as no assessments of gene expression in skin biopsies from the study participants have been conducted. Finally, other environmental factors that were not analyzed in the present study may also influence the risk of developing psoriasis.
5. Conclusions
The present study demonstrates, for the first time, that polymorphisms in the gene encoding glutathione S-transferase omega 1 serve as novel genetic markers for susceptibility to psoriasis. Notably, various polymorphisms in the GSTO1 gene exhibited synergistic effects on the risk of psoriasis and contributed to disease susceptibility in a sex-dependent manner. Additionally, we identified joint effects on disease risk for polymorphisms in the GSTO1 gene and the catalytic subunit of the glutamate cysteine ligase gene, which encodes the first enzyme in the cellular glutathione biosynthetic pathway. Furthermore, we observed that environmental risk factors, such as alcohol abuse and cigarette smoking, also exhibited joint effects with GSTO1 and GCLC polymorphisms on the risk of developing psoriasis. The results of the study provide new evidence confirming that psoriasis is a typical multifactorial disease, resulting from a complex interaction of genetic and environmental factors. In addition, this study emphasizes the crucial role of impaired glutathione detoxification mechanisms in the molecular pathogenesis of psoriasis. Potential mechanisms by which GSTO1 gene polymorphisms may contribute to the development of psoriasis are summarized in Figure 1. The phenotypic effects associated with GSTO1 gene polymorphisms are likely mediated by alterations—specifically reductions—in the expression levels of the GSTO1 and GSTO2 genes, as well as other adjacent genes that may be regulated by shared enhancers located within the genomic region 10q25.1. Polymorphisms in GSTO1 and GSTO2 are known to be sites for alternative splicing of these genes in the skin. This may lead to the production of functionally distinct enzyme isoforms with varying activities concerning the conjugation of cutaneous xenobiotics and free radicals with glutathione. Increased consumption of glutathione by glutathione S-transferases and glutathione peroxidases, which neutralize free radicals and xenobiotics, along with the harmful effects of alcohol, smoking, and other external factors, contributes to the depletion of glutathione, in addition to its reduced synthesis by glutamate cysteine ligase. Consequently, these disorders lead to the accumulation of free radicals and toxic substances, thereby initiating oxidative stress and causing damage to skin tissue. It is unequivocal that oxidative stress acts as a mechanistic pathway in the pathogenesis of psoriasis and represents a fundamental mechanism underlying the detrimental effects of environmental chemicals [9]. Gaining deeper insights into how the polymorphisms of the GSTO1 gene interact with other genes associated with glutathione metabolism could pave the way for innovative, evidence-based strategies for treating and preventing psoriasis.
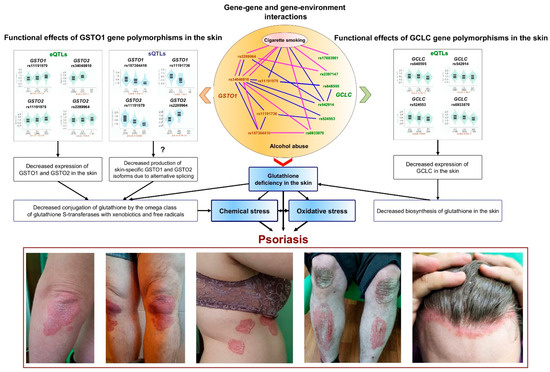
Figure 1.
Proposed mechanisms for the involvement of GSTO1 and GCLC gene polymorphisms in the pathogenesis of psoriasis.
The central section of the figure provides a schematic representation of the interactions between single nucleotide polymorphisms and their relationships with environmental factors, specifically alcohol abuse and cigarette smoking. These factors are the most significantly correlated with the risk of psoriasis, as determined by the top five n-th-order mbmdr models. The blue lines in the central section represent interactions among males, while the pink lines indicate interactions among females. The left and right sections of the figure display violin plots of expression quantitative trait loci (eQTLs) and splicing quantitative trait loci (sQTLs) for the studied SNPs obtained from the GTEx portal (https://www.gtexportal.org/home/, accessed on 8 December 2024). Below the plots are photographs of patients with psoriasis from our own collection. Comments regarding the figure are provided in the main text of the article.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jox15020060/s1, Supplementary Table S1: Associations between the polymorphisms of the GSTO1 gene and psoriasis risk in a population of the UK Biobank; Supplementary Table S2: GSTO1 genotype combinations and their associations with psoriasis risk; Supplementary Table S3: GSTO1 by GCLC genotype combinations and their associations with psoriasis risk in the entire group; Supplementary Table S4: The best n-th-order mbmdr models of SNP × risk factor interactions significantly associated with psoriasis risk in the entire group.
Author Contributions
Conceptualization, A.P.; methodology, A.P., R.S., E.E., O.B. and M.C.; software, A.P. and M.S.; validation, A.P. and M.C.; formal analysis, A.P., R.S. and O.B.; investigation, A.P., R.S., E.E. and O.B.; resources, R.S. and E.E.; data curation, A.P. and M.S.; writing—original draft preparation, A.P. and M.S.; writing—review and editing, A.P., M.S. and M.C.; visualization, A.P.; supervision, A.P.; project administration, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Kursk State Medical University (protocol No. 4, 9 April 2018).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author due to sensitivity concerns.
Acknowledgments
We thank all patients with psoriasis, healthy volunteers, and hospital staff in the Kursk region for their participation in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Campanati, A.; Marani, A.; Martina, E.; Diotallevi, F.; Radi, G.; Offidani, A. Psoriasis as an Immune-Mediated and Inflammatory Systemic Disease: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 1511. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.; Ashcroft, D.M.; on behalf of the Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Capon, F. The Genetic Basis of Psoriasis. Int. J. Mol. Sci. 2017, 18, 2526. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, M.; Huang, H.; Li, X.; Qian, D.; Hong, X.; Zheng, L.; Hong, J.; Zhu, Z.; Zheng, X.; et al. Exome-Wide Rare Loss-of-Function Variant Enrichment Study of 21,347 Han Chinese Individuals Identifies Four Susceptibility Genes for Psoriasis. J. Investig. Dermatol. 2020, 140, 799–805.e1. [Google Scholar] [CrossRef]
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347. [Google Scholar] [CrossRef] [PubMed]
- Lønnberg, A.S.; Skov, L.; Skytthe, A.; Kyvik, K.O.; Pedersen, O.B.; Thomsen, S.F. Heritability of psoriasis in a large twin sample. Br. J. Dermatol. 2013, 169, 412–416. [Google Scholar] [CrossRef]
- Zeng, J.; Luo, S.; Huang, Y.; Lu, Q. Critical role of environmental factors in the pathogenesis of psoriasis. J. Dermatol. 2017, 44, 863–872. [Google Scholar] [CrossRef]
- Kathuria, S.; Puri, P.; Nandar, S.; Ramesh, V. Effects of air pollution on the skin: A review. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 415–423. [Google Scholar] [CrossRef]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Viera, M.S.; Marrot, L.; Chuberre, B.; Dreno, B. The impact of airborne pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Y.; Yang, J.; Tian, Y. Exposure to Air Pollution, Genetic Susceptibility, and Psoriasis Risk in the UK. JAMA Netw. Open 2024, 7, e2421665. [Google Scholar] [CrossRef] [PubMed]
- Liaw, F.-Y.; Chen, W.-L.; Kao, T.-W.; Chang, Y.-W.; Huang, C.-F. Exploring the link between cadmium and psoriasis in a nationally representative sample. Sci. Rep. 2017, 7, 1723. [Google Scholar] [CrossRef] [PubMed]
- Wacewicz-Muczyńska, M.; Socha, K.; Soroczyńska, J.; Niczyporuk, M.; Borawska, M.H. Cadmium, lead and mercury in the blood of psoriatic and vitiligo patients and their possible associations with dietary habits. Sci. Total. Environ. 2021, 757, 143967. [Google Scholar] [CrossRef] [PubMed]
- Bellinato, F.; Adami, G.; Vaienti, S.; Benini, C.; Gatti, D.; Idolazzi, L.; Fassio, A.; Rossini, M.; Girolomoni, G.; Gisondi, P. Association Between Short-term Exposure to Environmental Air Pollution and Psoriasis Flare. JAMA Dermatol. 2022, 158, 375–381. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Yang, R.; Yu, H.; Shang, S.; Hu, Y. Short-term exposure to ambient fine particulate matter and psoriasis: A time-series analysis in Beijing, China. Front. Public Health 2022, 10, 1015197. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, Z.; Shen, J.; Wu, Y.; Fang, L.; Xu, S.; Ma, Y.; Zhao, H.; Pan, F. Associations of exposure to blood and urinary heavy metal mixtures with psoriasis risk among U.S. adults: A cross-sectional study. Sci. Total. Environ. 2023, 887, 164133. [Google Scholar] [CrossRef]
- Götz, C.; Pfeiffer, R.; Tigges, J.; Blatz, V.; Jäckh, C.; Freytag, E.; Fabian, E.; Landsiedel, R.; Merk, H.F.; Krutmann, J.; et al. Xenobiotic metabolism capacities of human skin in comparison with a 3D epidermis model and keratinocyte-based cell culture as in vitro alternatives for chemical testing: Activating enzymes (Phase I). Exp. Dermatol. 2012, 21, 358–363. [Google Scholar] [CrossRef]
- van Eijl, S.; Zhu, Z.; Cupitt, J.; Gierula, M.; Götz, C.; Fritsche, E.; Edwards, R.J. Elucidation of xenobiotic metabolism pathways in human skin and human skin models by proteomic profiling. PLoS ONE 2012, 7, e41721. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione Transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Cho, J.-W.; Ryu, J.; Park, S.G.; Park, B.C.; Choe, M.; Lee, K.-S. Proteomic analysis of psoriatic skin tissue for identification of differentially expressed proteins: Up-regulation of GSTP1, SFN and PRDX2 in psoriatic skin. Int. J. Mol. Med. 2011, 28, 785–792. [Google Scholar] [CrossRef]
- Karadag, A.S.; Uzunçakmak, T.K.; Ozkanli, S.; Oguztuzun, S.; Moran, B.; Akbulak, O.; Ozlu, E.; Zemheri, I.E.; Bilgili, S.G.; Akdeniz, N. An investigation of cytochrome p450 (CYP) and glutathione S-transferase (GST) isoenzyme protein expression and related interactions with phototherapy in patients with psoriasis vulgaris. Int. J. Dermatol. 2016, 56, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Akbulak, O.; Karadag, A.S.; Akdeniz, N.; Ozkanli, S.; Ozlu, E.; Zemheri, E.; Oguztuzun, S. Evaluation of oxidative stress via protein expression of glutathione S-transferase and cytochrome p450 (CYP450) ısoenzymes in psoriasis vulgaris patients treated with methotrexate. Cutan. Ocul. Toxicol. 2017, 37, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Zipprich, S.; Fuchs, T.; Neumann, C.; Westphal, G.; Schulz, T.; Müller, M.; Hallier, E. Combined Combined analysis of poly-morphisms of the tumor necrosis factor-alpha and interleukin-10 promoter regions and polymorphic xenobiotic metabolizing enzymes in psoriasis. J. Investig. Dermatol. 1999, 113, 214–220. [Google Scholar] [CrossRef]
- Richter-Hintz, D.; Their, R.; Steinwachs, S.; Kronenberg, S.; Fritsche, E.; Sachs, B.; Wulferink, M.; Tonn, T.; Esser, C. Allelic variants of drug metabolizing enzymes as risk factors in psoriasis. J. Investig. Dermatol. 2003, 120, 765–770. [Google Scholar] [CrossRef][Green Version]
- Gambichler, T.; Kreuter, A.; Susok, L.; Skrygan, M.; Rotterdam, S.; Höxtermann, S.; Müller, M.; Tigges, C.; Altmeyer, P.; Lahner, N. Glutathione-S-transferase T1 genotyping and phenotyping in psoriasis patients receiving treatment with oral fumaric acid esters. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Solak, B.; Karkucak, M.; Turan, H.; Ocakoğlu, G.; Sağ, Ş.Ö.; Uslu, E.; Yakut, T.; Erdem, T. Glutathione S-Transferase M1 and T1 Gene Polymorphisms in Patients with Chronic Plaque-Type Psoriasis: A Case-Control Study. Med Princ. Pract. 2016, 25, 155–158. [Google Scholar] [CrossRef]
- Hruska, P.; Rybecka, S.; Novak, J.; Zlamal, F.; Splichal, Z.; Slaby, O.; Vasku, V.; Bienertova-Vasku, J. Combinations of common polymorphisms within GSTA1 and GSTT1 as a risk factor for psoriasis in a central European population: A case-control study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e461–e463. [Google Scholar] [CrossRef]
- Jain, V.K.; Srivastava, D.S.L.; Verma, P.; Yadav, J.P. Polymorphism of glutathione S-transferase M1 and T1 genes and susceptibility to psoriasis disease: A study from North India. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 39–44. [Google Scholar] [CrossRef]
- Guarneri, F.; Sapienza, D.; Papaianni, V.; Marafioti, I.; Guarneri, C.; Mondello, C.; Roccuzzo, S.; Asmundo, A.; Cannavò, S.P. Association between genetic polymorphisms of glutathione S-transferase M1/T1 and psoriasis in a population from the area of the strict of messina (Southern Italy). Free. Radic. Res. 2020, 54, 57–63. [Google Scholar] [CrossRef]
- Tawfik, N.Z.; Abdallah, H.Y.; Abdullah, M.E.; Alshaarawy, H.F.; Atwa, M.A. Glutathione S-transferase M1 and T1 gene polymorphisms in psoriasis patients: A pilot case-control study. Egypt. J. Dermatol. Venerol. 2023, 43, 200–207. [Google Scholar] [CrossRef]
- Dursun, H.G.; Dursun, R.; Ayan, I.Ç.; Zamani, A.G.; Yıldırım, M.S. Relationship between Glutathione S-transferase gene polymorphisms and clinical features of psoriasis: A case-control study in the Turkish population. Turkderm 2024, 58, 75–82. [Google Scholar] [CrossRef]
- Klyosova, E.; Azarova, I.; Polonikov, A. A Polymorphism in the Gene Encoding Heat Shock Factor 1 (HSF1) Increases the Risk of Type 2 Diabetes: A Pilot Study Supports a Role for Impaired Protein Folding in Disease Pathogenesis. Life 2022, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, E.L.; Komkova, G.V.; Polonikova, A.A.; Churilin, M.I.; Solodilova, M.A. Relationship between polymorphism rs1546155 of the GGT7 gene and the risk of ischemic stroke. Res. Results Biomed. 2024, 10, 339–350. [Google Scholar] [CrossRef]
- Kobzeva, K.A.; Shilenok, I.V.; Belykh, A.E.; Gurtovoy, D.E.; Bobyleva, L.A.; Krapiva, A.B.; Stetskaya, T.A.; Bykanova, M.A.; Mezhenskaya, A.A.; Lysikova, E.A.; et al. C9orf16 (BBLN) gene, encoding a member of Hero proteins, is a novel marker in ischemic stroke risk. Res. Results Biomed. 2022, 8, 278–292. [Google Scholar] [CrossRef]
- Di Meglio, P.; Villanova, F.; Nestle, F.O. Psoriasis. Cold Spring Harb. Perspect. Med. 2014, 4, a015354. [Google Scholar] [CrossRef] [PubMed]
- Klyosova, E.Y.; Azarova, I.E.; Sunyaykina, O.A.; Polonikov, A.V. Validity of a brief screener for environmental risk factors of age-related diseases using type 2 diabetes and coronary artery disease as examples. Res. Results Biomed. 2022, 8, 130–137. [Google Scholar]
- Garbicz, J.; Całyniuk, B.; Górski, M.; Buczkowska, M.; Piecuch, M.; Kulik, A.; Rozentryt, P. Nutritional Therapy in Persons Suffering from Psoriasis. Nutrients 2021, 14, 119. [Google Scholar] [CrossRef]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.-W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef]
- Efanova, E.; Bushueva, O.; Saranyuk, R.; Surovtseva, A.; Churnosov, M.; Solodilova, M.; Polonikov, A. Polymorphisms of the GCLC Gene Are Novel Genetic Markers for Susceptibility to Psoriasis Associated with Alcohol Abuse and Cigarette Smoking. Life 2023, 13, 1316. [Google Scholar] [CrossRef]
- Azarova, I.; Klyosova, E.; Polonikov, A. Association between RAC1 gene variation, redox homeostasis and type 2 diabetes mellitus. Eur. J. Clin. Investig. 2022, 52, e13792. [Google Scholar] [CrossRef]
- Stetskaya, T.A.; Kursk State Medical University; Kobzeva, K.A.; Zaytsev, S.M.; Shilenok, I.V.; Komkova, G.V.; Goryainova, N.V.; Bushueva, O.Y.; Pavlov First Saint Petersburg State Medical University; Hospital, K.C.C.E. HSPD1 gene polymorphism is associated with an increased risk of ischemic stroke in smokers. Res. Results Biomed. 2024, 10, 175–186. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Calle, M.L.; Urrea, V.; Malats, N.; Van Steen, K. mbmdr: An R package for exploring gene-gene interactions associated with binary or quantitative traits. Bioinformatics 2010, 26, 2198–2199. [Google Scholar] [CrossRef][Green Version]
- Polonikov, A.; Bocharova, I.; Azarova, I.; Klyosova, E.; Bykanova, M.; Bushueva, O.; Polonikova, A.; Churnosov, M.; Solodilova, M. The Impact of Genetic Polymorphisms in Glutamate-Cysteine Ligase, a Key Enzyme of Glutathione Biosynthesis, on Ischemic Stroke Risk and Brain Infarct Size. Life 2022, 12, 602. [Google Scholar] [CrossRef]
- Zhabin, S.; Lazarenko, V.; Azarova, I.; Klyosova, E.; Bykanova, M.; Chernousova, S.; Bashkatov, D.; Gneeva, E.; Polonikova, A.; Churnosov, M.; et al. The Joint Link of the rs1051730 and rs1902341 Polymorphisms and Cigarette Smoking to Peripheral Artery Disease and Atherosclerotic Lesions of Different Arterial Beds. Life 2023, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.D.; Hahn, L.W.; Roodi, N.; Bailey, L.R.; Dupont, W.D.; Parl, F.F.; Moore, J.H. Multifactor-Dimensionality Reduction Reveals High-Order Interactions among Estrogen-Metabolism Genes in Sporadic Breast Cancer. Am. J. Hum. Genet. 2001, 69, 138–147. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Lu, T.-P. Gene-gene interaction: The curse of dimensionality. Ann. Transl. Med. 2019, 7, 813. [Google Scholar] [CrossRef]
- Lazarenko, V.; Churilin, M.; Azarova, I.; Klyosova, E.; Bykanova, M.; Ob’Edkova, N.; Churnosov, M.; Bushueva, O.; Mal, G.; Povetkin, S.; et al. Comprehensive Statistical and Bioinformatics Analysis in the Deciphering of Putative Mechanisms by Which Lipid-Associated GWAS Loci Contribute to Coronary Artery Disease. Biomedicines 2022, 10, 259. [Google Scholar] [CrossRef]
- Sharma, R.; Yang, Y.; Sharma, A.; Awasthi, S.; Awasthi, Y.C. Antioxidant role of glutathione S-transferases: Protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxidants Redox Signal. 2004, 6, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015, 289, 361–370. [Google Scholar] [CrossRef]
- Kim, Y.; Cha, S.J.; Choi, H.-J.; Kim, K. Omega Class Glutathione S-Transferase: Antioxidant Enzyme in Pathogenesis of Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 5049532. [Google Scholar] [CrossRef]
- Board, P.G.; Menon, D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 3267–3288. [Google Scholar] [CrossRef]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Coll, R.; O’Neill, L.A.; Board, P.G. Glutathione transferase Omega 1 is required for the lipopolysaccharide-stimulated induction of NADPH oxidase 1 and the production of reactive oxygen species in macrophages. Free. Radic. Biol. Med. 2014, 73, 318–327. [Google Scholar] [CrossRef]
- Yang, S.; Yan, K.-L.; Zhang, X.-J.; Xiao, F.-L.; Fan, X.; Gao, M.; Cui, Y.; Wang, P.-G.; Zhang, G.-L.; Sun, L.-D.; et al. Systematic evaluation of association between the microsomal glutathione S-transferase 2 common variation and psoriasis vulgaris in Chinese population. Arch. Dermatol. Res. 2006, 298, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Mazzilli, S.; Di Prete, M.; Dattola, A.; Cosio, T.; Barbato, D.L.; Costanza, G.; Lanna, C.; Manfreda, V.; Schumak, R.G.; et al. The Role of Glutathione-S Transferase in Psoriasis and Associated Comorbidities and the Effect of Dimethyl Fumarate in This Pathway. Front. Med. 2022, 9, 760852. [Google Scholar] [CrossRef] [PubMed]
- Farkas, Á.; Kemény, L. Alcohol, liver, systemic inflammation and skin: A focus on patients with psoriasis. Ski. Pharmacol. Physiol. 2013, 26, 119–126. [Google Scholar] [CrossRef]
- Dobozy, A.; Farkas, Á.; Kemény, L.; Széll, M.; Bata-Csörgő, Z. Ethanol and acetone stimulate the proliferation of HaCaT keratinocytes: The possible role of alcohol in exacerbating psoriasis. Arch. Dermatol. Res. 2003, 295, 56–62. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol. Res. Health 2003, 27, 277–284. [Google Scholar]
- Michalak, A.; Lach, T.; Cichoż-Lach, H. Oxidative Stress—A Key Player in the Course of Alcohol-Related Liver Disease. J. Clin. Med. 2021, 10, 3011. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Ishigaki, K.; Suzuki, A.; Tsuchida, Y.; Tsuchiya, H.; Sumitomo, S.; Nagafuchi, Y.; Miya, F.; Tsunoda, T.; Shoda, H.; et al. Splicing QTL analysis focusing on coding sequences reveals mechanisms for disease susceptibility loci. Nat. Commun. 2022, 13, 4659. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.E.; Akhtari, F.S.; Potter, T.A.; Fargo, D.C.; Schmitt, C.P.; Schurman, S.H.; Eccles, K.M.; Motsinger-Reif, A.; Hall, J.E.; Messier, K.P. The skin is no barrier to mixtures: Air pollutant mixtures and reported psoriasis or eczema in the Personalized Environment and Genes Study (PEGS). J. Expo. Sci. Environ. Epidemiology 2023, 33, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Mufti, A.; Maliyar, K.; Lytvyn, Y.; Yeung, J. Hydroxychloroquine effects on psoriasis: A systematic review and a cautionary note for COVID-19 treatment. J. Am. Acad. Dermatol. 2020, 83, 579–586. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Jafferany, M. Lithium and Psoriasis: What primary care and family physicians should know. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 435–439. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, M.; Zhao, C. Exposure to Per- and Polyfluoroalkyl Substances and Risk of Psoriasis: A Population-Based Study. Toxics 2024, 12, 828. [Google Scholar] [CrossRef]
- Zakharyan, R.A.; Sampayo-Reyes, A.; Healy, S.M.; Tsaprailis, G.; Board, P.G.; Liebler, D.C.; Aposhian, H.V. Human monomethylarsonic acid (MMA(V)) reductase is a member of the glutathione-S-transferase superfamily. Chem. Res. Toxicol. 2001, 14, 1051–1057. [Google Scholar] [CrossRef]
- Schmuck, E.M.; Board, P.G.; Whitbread, A.K.; Tetlow, N.; Cavanaugh, J.A.; Blackburn, A.C.; Masoumi, A. Characterization of the monomethylarsonate reductase and dehydroascorbate reductase activities of Omega class glutathione transferase variants: Implications for arsenic metabolism and the age-at-onset of Alzheimer’s and Parkinson’s diseases. Pharmacogenetics Genom. 2005, 15, 493–501. [Google Scholar] [CrossRef]
- Barnett, J.B.; Brundage, K.M. Immunotoxicology of pesticides and chemotherapies. In Comprehensive Toxicology, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2010; pp. 467–487. [Google Scholar]
- Kitahata, K.; Matsuo, K.; Hara, Y.; Naganuma, T.; Oiso, N.; Kawada, A.; Nakayama, T. Ascorbic acid derivative DDH-1 ameliorates psoriasis-like skin lesions in mice by suppressing inflammatory cytokine expression. J. Pharmacol. Sci. 2018, 138, 284–288. [Google Scholar] [CrossRef]
- Mittelstrass, K.; Ried, J.S.; Yu, Z.; Krumsiek, J.; Gieger, C.; Prehn, C.; Roemisch-Margl, W.; Polonikov, A.; Peters, A.; Theis, F.J.; et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLOS Genet. 2011, 7, e1002215. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.C.; Winkler, T.W.; Kutalik, Z.; Berndt, S.I.; Jackson, A.U.; Monda, K.L.; Kilpeläinen, T.O.; Esko, T.; Mägi, R.; Li, S.; et al. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLOS Genet. 2013, 9, e1003500. [Google Scholar] [CrossRef]
- Queiro, R.; Tejón, P.; Coto, P.; Alonso, S.; Alperi, M.; Sarasqueta, C.; González, S.; Martínez-Borra, J.; López-Larrea, C.; Ballina, J. Clinical differences between men and women with psoriatic arthritis: Relevance of the analysis of genes and polymorphisms in the major histocompatibility complex region and of the age at onset of psoriasis. J. Immunol. Res. 2013, 2013, 482691. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Wither, J.; Jurisica, I.; Chandran, V.; Eder, L. Sex differences in biomarkers and biologic mechanisms in psoriatic diseases and spondyloarthritis. J. Autoimmun. 2025, 152, 103394. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Mieczan, T.; Wójcik, G. Importance of Redox Equilibrium in the Pathogenesis of Psoriasis—Impact of Antioxidant-Rich Diet. Nutrients 2020, 12, 1841. [Google Scholar] [CrossRef]
- Chung, M.; Bartholomew, E.; Yeroushalmi, S.; Hakimi, M.; Bhutani, T.; Liao, W. Dietary Intervention and Supplements in the Management of Psoriasis: Current Perspectives. Psoriasis Targets Ther. 2022, 12, 151–176. [Google Scholar] [CrossRef]
- Vogt, B.L.; Richie, J.P. Glutathione depletion and recovery after acute ethanol administration in the aging mouse. Biochem. Pharmacol. 2007, 73, 1613–1621. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fares-Medina, S.; Díaz-Caro, I.; García-Montes, R.; Corral-Liria, I.; García-Gómez-Heras, S. Multiple Chemical Sensitivity Syndrome: First Symptoms and Evolution of the Clinical Picture: Case-Control Study/Epidemiological Case-Control Study. Int. J. Environ. Res. Public Health 2022, 19, 15891. [Google Scholar] [CrossRef]
- Chhabra, R.S.; Fouts, J.R. Sex differences in the metabolism of xenobiotics by extrahepatic tissue in rats. Drug Metab. Dispos. 1974, 2, 375–379. [Google Scholar] [CrossRef]
- Mugford, C.A.; Kedderis, G.L. Sex-dependent metabolism of xenobiotics. Drug Metab. Rev. 1998, 30, 441–498. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).