Pregnenolone 16-Alpha Carbonitrile, an Agonist of Rodent Pregnane X Receptor, Regulates Testosterone Biosynthesis in Rodent Leydig Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results
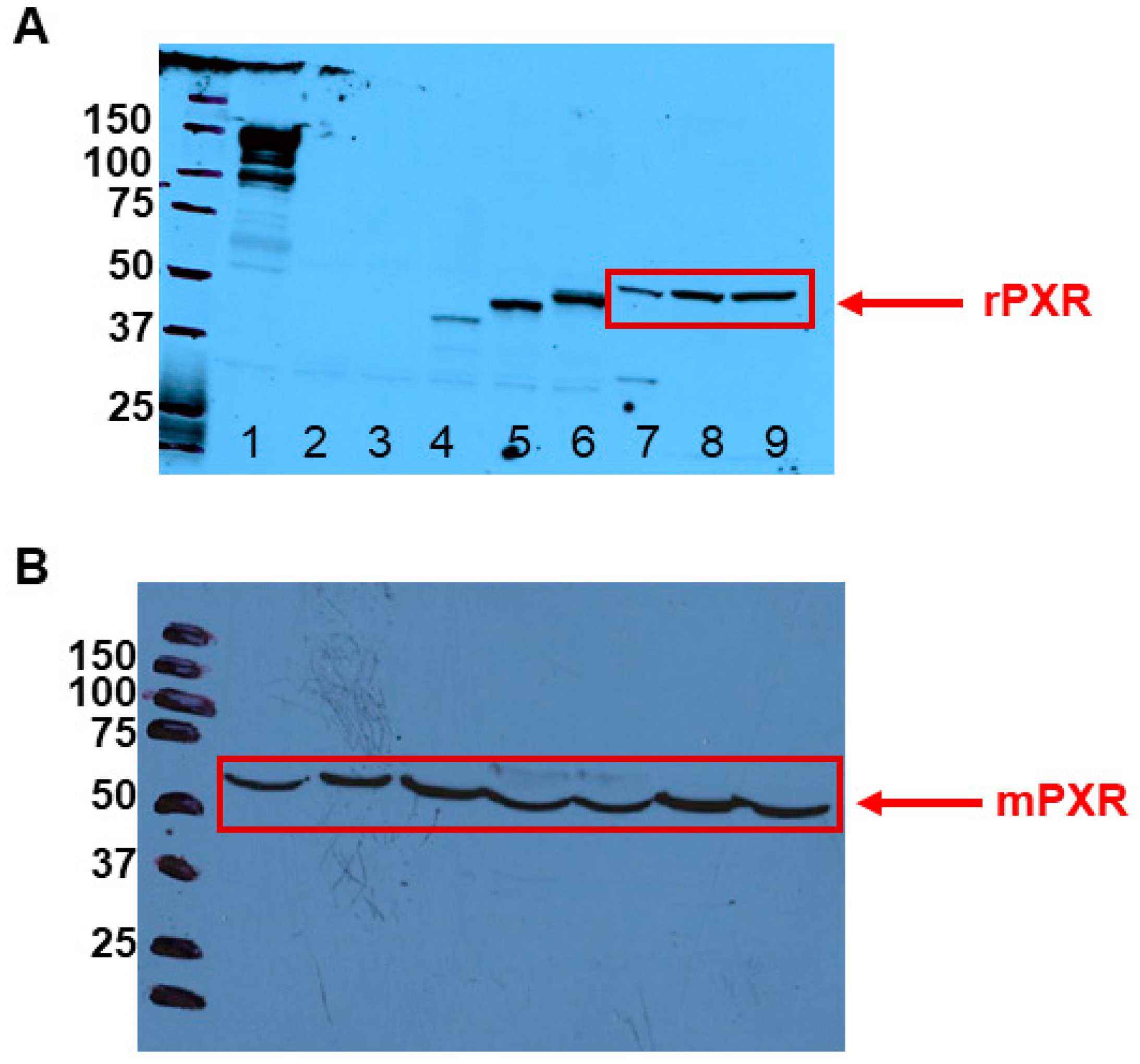
3.1. PXR Is Expressed in Rodent LCs
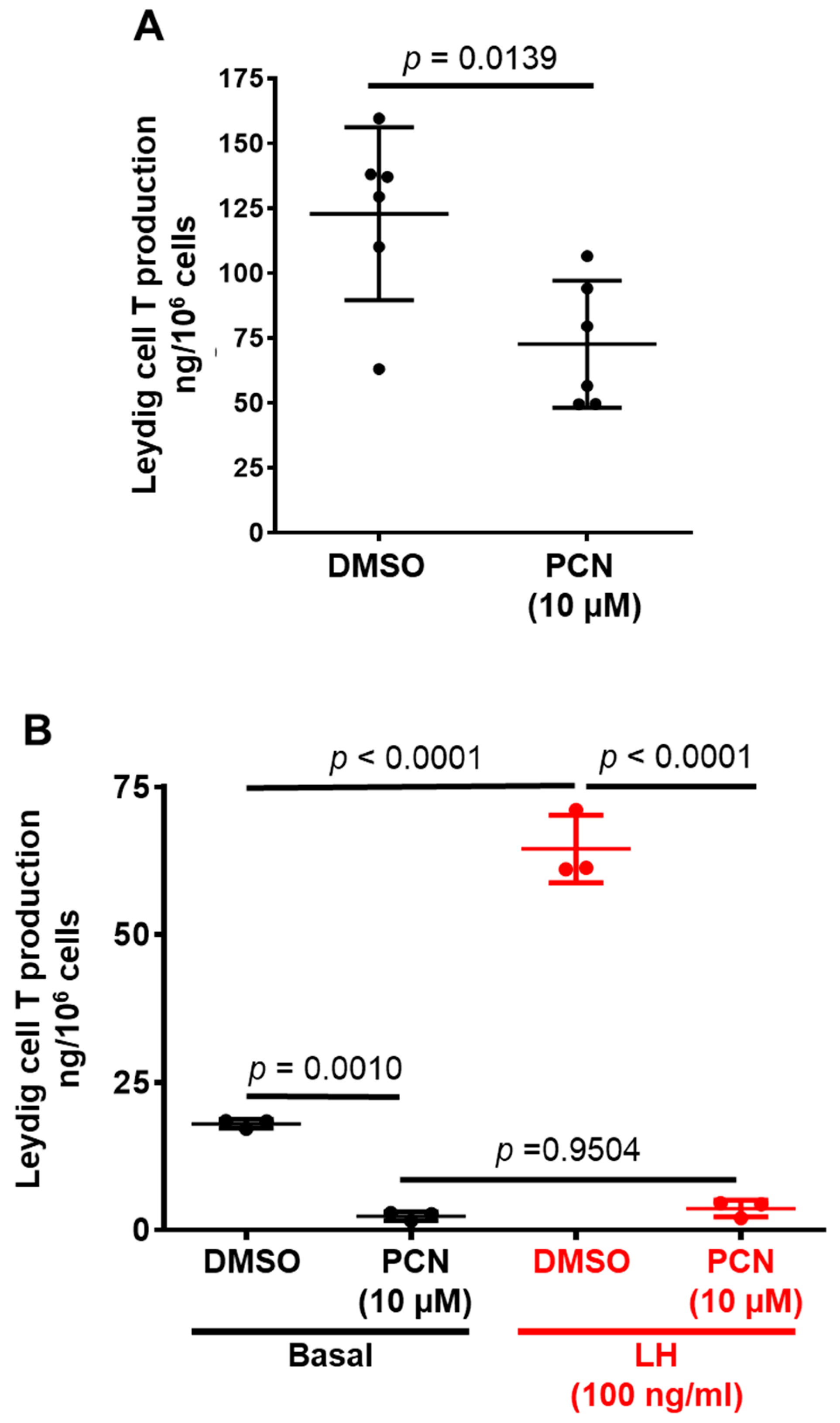
3.2. Pharmacological Activation of rPXR Regulates T Secretion by LCs
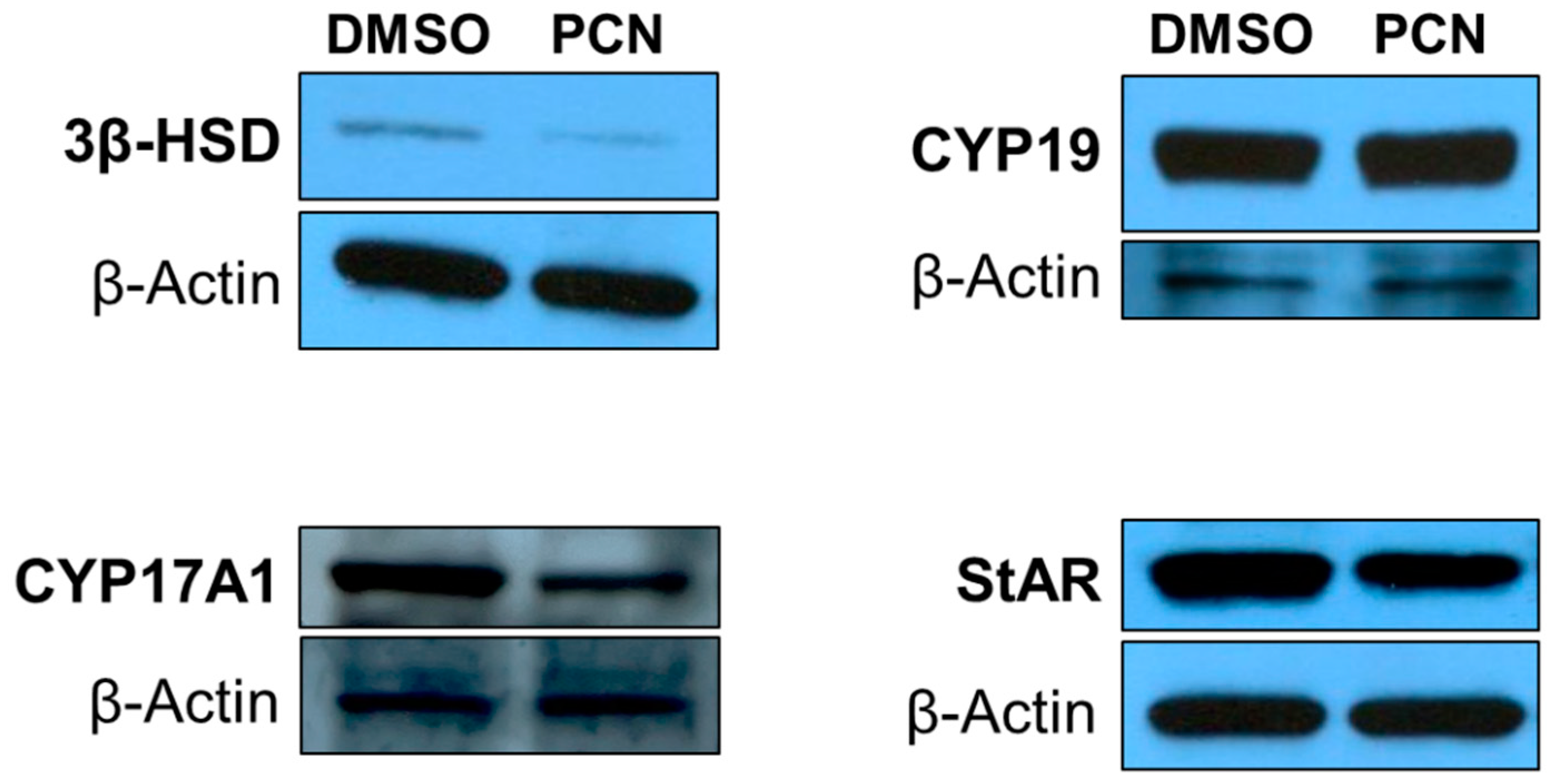
3.3. Pharmacological Activation of rPXR Affects Protein Expression of Key Steroidogenic Enzymes Involved in T Biosynthesis
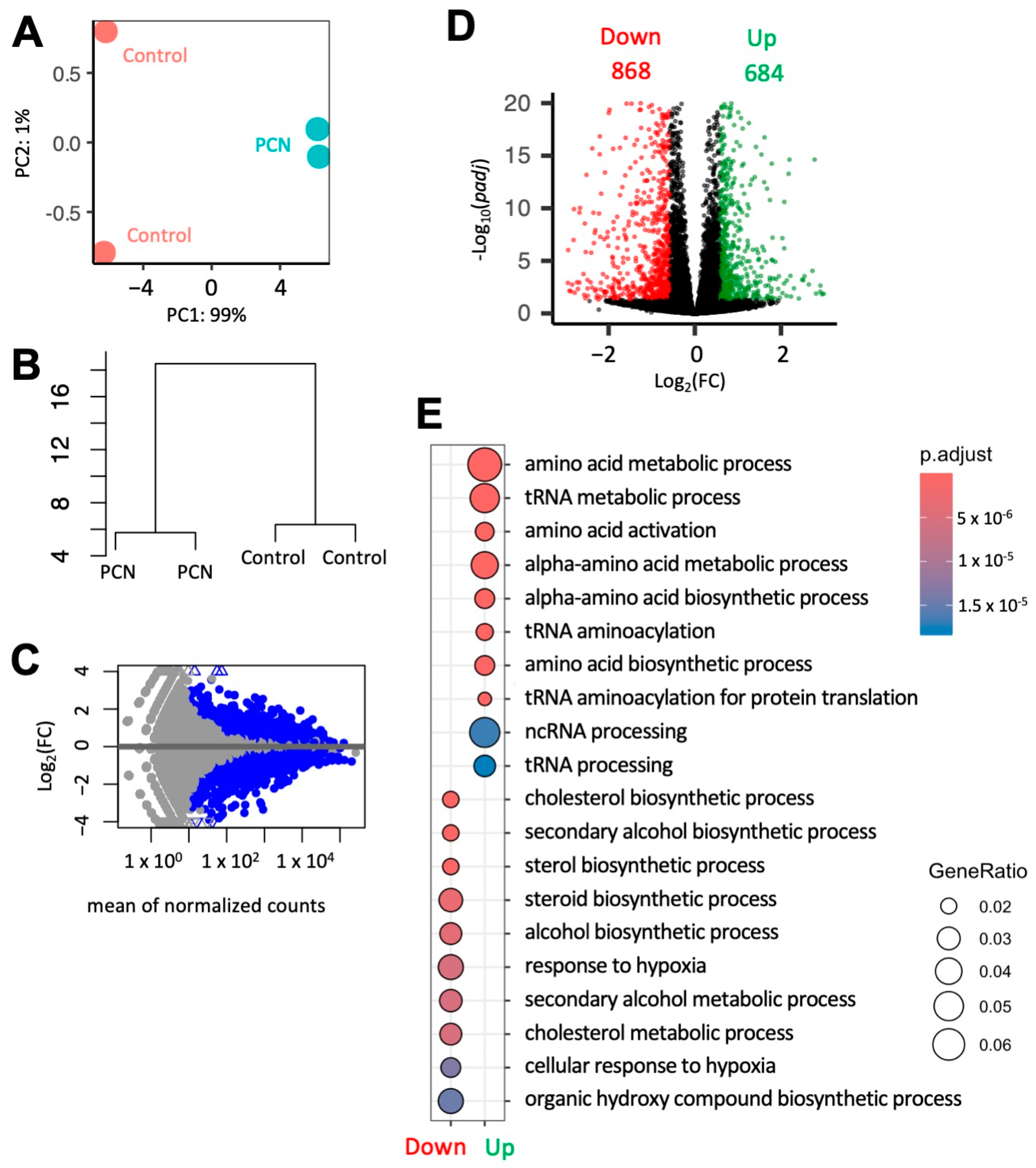
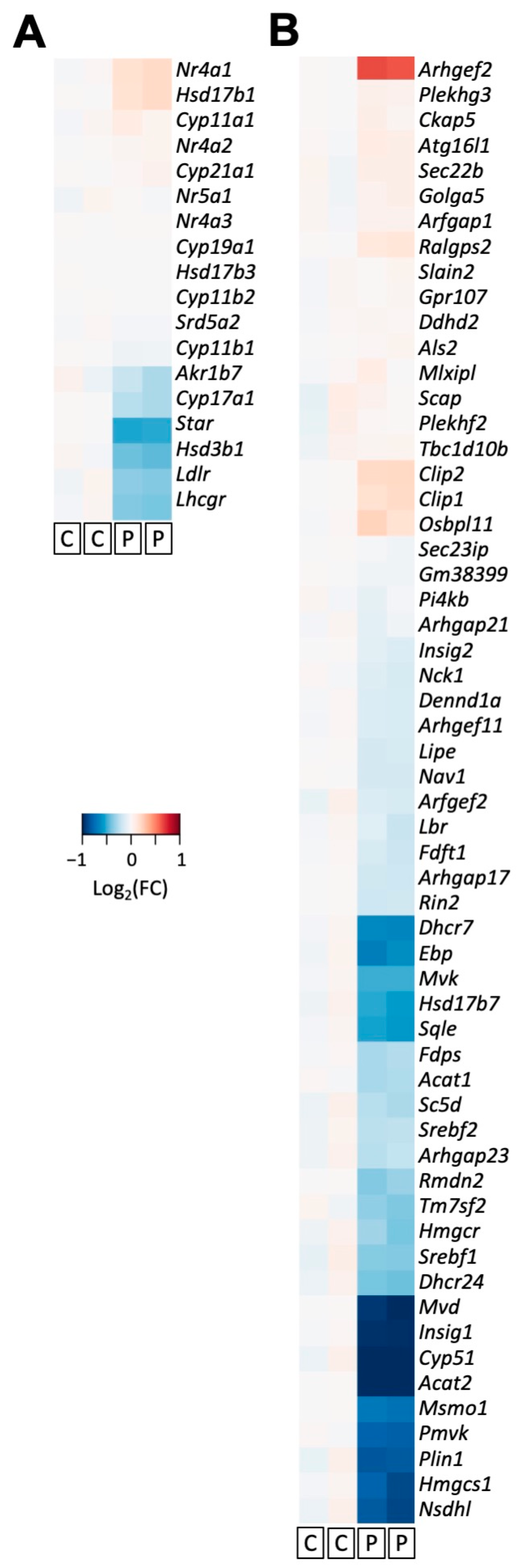
3.4. PCN Induces Changes in Gene Expression of Key Players Involved in Steroidogenesis in Mouse MA-10 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyagi, V.; Scordo, M.; Yoon, R.S.; Liporace, F.A.; Greene, L.W. Revisiting the role of testosterone: Are we missing something? Rev. Urol. 2017, 19, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bammel, A.; van der Mee, K.; Ohnhaus, E.E.; Kirch, W. Divergent effects of different enzyme-inducing agents on endogenous and exogenous testosterone. Eur. J. Clin. Pharmacol. 1992, 42, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Birzniece, V.; Sata, A.; Sutanto, S.; Ho, K.K. Neuroendocrine regulation of growth hormone and androgen axes by selective estrogen receptor modulators in healthy men. J. Clin. Endocrinol. Metab. 2010, 95, 5443–5448. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jia, Y.; Chong, L.; Jiang, J.; Yang, Y.; Li, L.; Ma, A.; Sun, Z.; Zhou, L. Effects of oral cimetidine on the reproductive system of male rats. Exp. Ther. Med. 2018. [Google Scholar] [CrossRef]
- Gupta, C.; Yaffe, S.; Shapiro, B. Prenatal exposure to phenobarbital permanently decreases testosterone and causes reproductive dysfunction. Science 1982, 216, 640–642. [Google Scholar] [CrossRef]
- Dolatabadi, A.A.; Zarchii, S.R. The effect of prescription of different Dexamethasone doses on reproductive system. Biomed. Res. 2015, 26, 656–660. [Google Scholar]
- Verrotti, A.; Loiacono, G.; Laus, M.; Coppola, G.; Chiarelli, F.; Tiboni, G.M. Hormonal and reproductive disturbances in epileptic male patients: Emerging issues. Reprod. Toxicol. 2011, 31, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; DeVane, C.L.; Lewis, J.G.; Wang, J.S.; Ruan, Y.; Chavin, K.D.; Markowitz, J.S. Effects of St John’s wort (Hypericum perforatum L.) extract on plasma androgen concentrations in healthy men and women: A pilot study. Phytother. Res. 2005, 19, 901–906. [Google Scholar] [CrossRef]
- Armanini, D.; Bonanni, G.; Mattarello, M.J.; Fiore, C.; Sartorato, P.; Palermo, M. Licorice consumption and serum testosterone in healthy man. Exp. Clin. Endocrinol. Diabetes 2003, 111, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.Y.; Pu, H.F.; Kaphle, K.; Lin, S.F.; Wu, L.S.; Lin, J.H.; Tsai, Y.F. Ginkgo biloba extract enhances male copulatory behavior and reduces serum prolactin levels in rats. Horm. Behav. 2008, 53, 225–231. [Google Scholar] [CrossRef]
- Ohno, S.; Nakajima, Y.; Inoue, K.; Nakazawa, H.; Nakajin, S. Genistein administration decreases serum corticosterone and testosterone levels in rats. Life Sci. 2003, 74, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamaa, P.; Zhang, F.-P.; Saarinen, L.; Mikkonen, L.; Hautaniemi, S.; Jänne, O.A. The Phytoestrogen Genistein Is a Tissue-Specific Androgen Receptor Modulator. Endocrinology 2011, 152, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- Jeminiwa, B.O.; Knight, R.M.; Braden, T.D.; Cruz-Espindola, C.; Boothe, D.M.; Akingbemi, B.T. Regulation of the neuroendocrine axis in male rats by soy-based diets is independent of age and due specifically to isoflavone actiondagger. Biol. Reprod. 2020, 103, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, P.; Sridhar, M.; Dhanammal, S.; Vijayababu, M.R.; Arunkumar, A.; Srinivasan, N.; Arunakaran, J. Effects of vitamin supplementation on PCB (Aroclor 1254)-induced changes in ventral prostatic androgen and estrogen receptors. Endocr. Res. 2004, 30, 469–480. [Google Scholar] [CrossRef]
- Nanjappa, M.K.; Simon, L.; Akingbemi, B.T. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol. Reprod. 2012, 86, 135. [Google Scholar] [CrossRef]
- Della Seta, D.; Minder, I.; Belloni, V.; Aloisi, A.M.; Dessi-Fulgheri, F.; Farabollini, F. Pubertal exposure to estrogenic chemicals affects behavior in juvenile and adult male rats. Horm. Behav. 2006, 50, 301–307. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, H.; Liu, Y.; Xu, M.; Dai, J. Alterations in gene expression and testosterone synthesis in the testes of male rats exposed to perfluorododecanoic acid. Toxicol. Sci. 2007, 98, 206–215. [Google Scholar] [CrossRef]
- Shi, Z.; Ding, L.; Zhang, H.; Feng, Y.; Xu, M.; Dai, J. Chronic exposure to perfluorododecanoic acid disrupts testicular steroidogenesis and the expression of related genes in male rats. Toxicol. Lett. 2009, 188, 192–200. [Google Scholar] [CrossRef]
- Zorrilla, L.M.; Gibson, E.K.; Jeffay, S.C.; Crofton, K.M.; Setzer, W.R.; Cooper, R.L.; Stoker, T.E. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol. Sci. 2009, 107, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Pan, C.; Hu, J.X.; Li, J.; Xu, L.C. Effects of cypermethrin on male reproductive system in adult rats. Biomed. Environ. Sci. 2013, 26, 201–208. [Google Scholar] [CrossRef]
- Lafuente, A.; Marquez, N.; Pousada, Y.; Pazo, D.; Esquifino, A.I. Possible estrogenic and/or antiandrogenic effects of methoxychlor on prolactin release in male rats. Arch. Toxicol. 2000, 74, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Jeminiwa, B.O.; Knight, R.C.; Abbot, K.L.; Pondugula, S.R.; Akingbemi, B.T. Gonadal sex steroid hormone secretion after exposure of male rats to estrogenic chemicals and their combinations. Mol. Cell. Endocrinol. 2021, 533, 111332. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Moore, L.B.; Shenk, J.L.; Wisely, G.B.; Hamilton, G.A.; McKee, D.D.; Tomkinson, N.C.; LeCluyse, E.L.; Lambert, M.H.; Willson, T.M.; et al. The pregnane X receptor: A promiscuous xenobiotic receptor that has diverged during evolution. Mol. Endocrinol. 2000, 14, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Moore, J.T.; Wade, L.; Staudinger, J.L.; Watson, M.A.; Jones, S.A.; McKee, D.D.; Oliver, B.B.; Willson, T.M.; Zetterstrom, R.H.; et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 1998, 92, 73–82. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Guo, C.; Wang, J.; Boral, D.; Nie, D. Nuclear receptors in the multidrug resistance through the regulation of drug-metabolizing enzymes and drug transporters. Biochem. Pharmacol. 2012, 83, 1112–1126. [Google Scholar] [CrossRef]
- Staudinger, J.L.; Goodwin, B.; Jones, S.A.; Hawkins-Brown, D.; MacKenzie, K.I.; LaTour, A.; Liu, Y.; Klaassen, C.D.; Brown, K.K.; Reinhard, J.; et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 3369–3374. [Google Scholar] [CrossRef]
- Kodama, S.; Moore, R.; Yamamoto, Y.; Negishi, M. Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem. J. 2007, 407, 373–381. [Google Scholar] [CrossRef]
- Zhou, J.; Zhai, Y.; Mu, Y.; Gong, H.; Uppal, H.; Toma, D.; Ren, S.; Evans, R.M.; Xie, W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. 2006, 281, 15013–15020. [Google Scholar] [CrossRef]
- Nakamura, K.; Moore, R.; Negishi, M.; Sueyoshi, T. Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J. Biol. Chem. 2007, 282, 9768–9776. [Google Scholar] [CrossRef]
- Zhai, Y.; Pai, H.V.; Zhou, J.; Amico, J.A.; Vollmer, R.R.; Xie, W. Activation of pregnane X receptor disrupts glucocorticoid and mineralocorticoid homeostasis. Mol. Endocrinol. 2007, 21, 138–147. [Google Scholar] [CrossRef]
- Zhang, B.; Cheng, Q.; Ou, Z.; Lee, J.H.; Xu, M.; Kochhar, U.; Ren, S.; Huang, M.; Pflug, B.R.; Xie, W. Pregnane X receptor as a therapeutic target to inhibit androgen activity. Endocrinology 2010, 151, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Abbott, K.L.; Chaudhury, C.S.; Chandran, A.; Vishveshwara, S.; Dvorak, Z.; Jiskrova, E.; Poulikova, K.; Vyhlidalova, B.; Mani, S.; Pondugula, S.R. Belinostat, at Its Clinically Relevant Concentrations, Inhibits Rifampicin-Induced CYP3A4 and MDR1 Gene Expression. Mol. Pharmacol. 2019, 95, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Pondugula, S.R.; Flannery, P.C.; Abbott, K.L.; Coleman, E.S.; Mani, S.; Temesgen, S.; Xie, W. Diindolylmethane, a naturally occurring compound, induces CYP3A4 and MDR1 gene expression by activating human PXR. Toxicol. Lett. 2015, 232, 580–589. [Google Scholar] [CrossRef]
- Pondugula, S.R.; Flannery, P.C.; Apte, U.; Babu, J.R.; Geetha, T.; Rege, S.D.; Chen, T.; Abbott, K.L. Mg2+/Mn2+-Dependent Phosphatase 1A Is Involved in Regulating Pregnane X Receptor-Mediated Cytochrome p450 3A4 Gene Expression. Drug Metab. Dispos. 2015, 43, 385–391. [Google Scholar] [CrossRef]
- Cochran, R.C.; Ewing, L.L.; Niswender, G.D. Serum levels of follicle stimulating hormone, luteinizing hormone, prolactin, testosterone, 5 alpha-dihydrotestosterone, 5 alpha-androstane-3 alpha, 17 beta-diol, 5 alpha-androstane-3 beta, 17 beta-diol, and 17 beta-estradiol from male beagles with spontaneous or induced benign prostatic hyperplasia. Investig. Urol. 1981, 19, 142–147. [Google Scholar]
- Shrivastav, T.G.; Kanaujia, P.K. Direct radioimmunoassay for the measurement of serum testosterone using 3H as label. J. Immunoassay Immunochem. 2007, 28, 127–136. [Google Scholar] [CrossRef]
- Ascoli, M. Characterization of several clonal lines of cultured Leydig tumor cells: Gonadotropin receptors and steroidogenic responses. Endocrinology 1981, 108, 88–95. [Google Scholar] [CrossRef]
- Flannery, P.C.; Abbott, K.L.; Pondugula, S.R. Correlation of PPM1A Downregulation with CYP3A4 Repression in the Tumor Liver Tissue of Hepatocellular Carcinoma Patients. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 297–304. [Google Scholar] [CrossRef]
- Zheng, H.S.; Daniel, J.G.; Salamat, J.M.; Mackay, L.; Foradori, C.D.; Kemppainen, R.J.; Pondugula, S.R.; Tao, Y.X.; Huang, C.J. Early transcriptomic response of mouse adrenal gland and Y-1 cells to dexamethasone. Endocr. Connect. 2022, 11, e220064. [Google Scholar] [CrossRef]
- Kotiya, D.; Jaiswal, B.; Ghose, S.; Kaul, R.; Datta, K.; Tyagi, R.K. Role of PXR in Hepatic Cancer: Its Influences on Liver Detoxification Capacity and Cancer Progression. PLoS ONE 2016, 11, e0164087. [Google Scholar] [CrossRef]
- Pondugula, S.R.; Ferniany, G.; Ashraf, F.; Abbott, K.L.; Smith, B.F.; Coleman, E.S.; Mansour, M.; Bird, R.C.; Smith, A.N.; Karthikeyan, C.; et al. Stearidonic acid, a plant-based dietary fatty acid, enhances the chemosensitivity of canine lymphoid tumor cells. Biochem. Biophys. Res. Commun. 2015, 460, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hoque, M.T.; Jenabian, M.-A.; Vyboh, K.; Whyte, S.-K.; Sheehan, N.L.; Brassard, P.; Bélanger, M.; Chomont, N.; Fletcher, C.V.; et al. Antiretroviral drug transporters and metabolic enzymes in human testicular tissue: Potential contribution to HIV-1 sanctuary site. J. Antimicrob. Chemother. 2016, 71, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Whyte-Allman, S.-K.; Hoque, M.T.; Jenabian, M.-A.; Routy, J.-P.; Bendayan, R. Xenobiotic Nuclear Receptors Pregnane X Receptor and Constitutive Androstane Receptor Regulate Antiretroviral Drug Efflux Transporters at the Blood-Testis Barrier. J. Pharmacol. Exp. Ther. 2017, 363, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.A.; Squires, E.J. Effects of nuclear receptor transactivation on steroid hormone synthesis and gene expression in porcine Leydig cells. J. Steroid Biochem. Mol. Biol. 2013, 133, 93–100. [Google Scholar] [CrossRef]
- Tian, M.; Huang, Q.; Wang, H.; Martin, F.L.; Liu, L.; Zhang, J.; Shen, H. Biphasic effects of perfluorooctanoic acid on steroidogenesis in mouse Leydig tumour cells. Reprod. Toxicol. 2019, 83, 54–62. [Google Scholar] [CrossRef]
| Target Protein | Dilution | Host Species | Catalog No/Manufacturer or Reference |
|---|---|---|---|
| PXR | 3000 | Rabbit polyclonal | Provided by Dr. Rakesh Tyagi [40] |
| β-actin | 2000 | Goat polyclonal | sc-1616, Santa Cruz Biotechnologies |
| CYP17A1 | 20,000 | Goat polyclonal | sc-46081, Santa Cruz Biotechnologies |
| 3β-HSD | 2000 | Rabbit polyclonal | sc-28206, Santa Cruz Biotechnologies |
| CYP19 | 500 | Rabbit polyclonal | sc-30086, Santa Cruz Biotechnologies |
| StAR | 5000 | Rabbit polyclonal | sc-25806, Santa Cruz Biotechnologies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamat, J.M.; Ayala, E.M.; Huang, C.-C.J.; Wilbanks, F.S.; Knight, R.C.; Akingbemi, B.T.; Pondugula, S.R. Pregnenolone 16-Alpha Carbonitrile, an Agonist of Rodent Pregnane X Receptor, Regulates Testosterone Biosynthesis in Rodent Leydig Cells. J. Xenobiot. 2024, 14, 1256-1267. https://doi.org/10.3390/jox14030071
Salamat JM, Ayala EM, Huang C-CJ, Wilbanks FS, Knight RC, Akingbemi BT, Pondugula SR. Pregnenolone 16-Alpha Carbonitrile, an Agonist of Rodent Pregnane X Receptor, Regulates Testosterone Biosynthesis in Rodent Leydig Cells. Journal of Xenobiotics. 2024; 14(3):1256-1267. https://doi.org/10.3390/jox14030071
Chicago/Turabian StyleSalamat, Julia M., Elizabeth M. Ayala, Chen-Che J. Huang, Frank S. Wilbanks, Rachel C. Knight, Benson T. Akingbemi, and Satyanarayana R. Pondugula. 2024. "Pregnenolone 16-Alpha Carbonitrile, an Agonist of Rodent Pregnane X Receptor, Regulates Testosterone Biosynthesis in Rodent Leydig Cells" Journal of Xenobiotics 14, no. 3: 1256-1267. https://doi.org/10.3390/jox14030071
APA StyleSalamat, J. M., Ayala, E. M., Huang, C.-C. J., Wilbanks, F. S., Knight, R. C., Akingbemi, B. T., & Pondugula, S. R. (2024). Pregnenolone 16-Alpha Carbonitrile, an Agonist of Rodent Pregnane X Receptor, Regulates Testosterone Biosynthesis in Rodent Leydig Cells. Journal of Xenobiotics, 14(3), 1256-1267. https://doi.org/10.3390/jox14030071







