Association between Liver and Kidney Function and Birth Outcomes in Pregnant Surinamese Women Exposed to Mercury and Lead in the Caribbean Consortium for Research in Environmental and Occupational Health (CCREOH) Environmental Epidemiologic Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Metals and Elements
2.3. Liver and Kidney Biomarkers
2.4. Birth Outcomes
2.5. Data Analysis
2.6. IRB
3. Results
4. ABOs in Relation with Liver and Kidney Function and Blood Hg and Pb Levels
5. Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Axelrad, D.; Adams, K.; Chowdhury, F.; D’Amico, L.; Douglass, E.; Hudson, G.; Koustas, E.; Lam, J.; Lorenz, A.; Miller, G.; et al. Environmental Protection Agency Adverse Birth Outcomes. Am. Child. Environ. 2013, 3, 264–388. [Google Scholar]
- WHO; UNAIDS; UNFPA; UNICEF; UN WOMEN; The World Bank Group. Survive, Thrive, Transform. Global Strategy for Women’s, Children’s and Adolescents’ Health: 2018 Report on Progress Towards 2030 Targets; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hassen, T.A.; Chojenta, C.; Egan, N.; Loxton, D. The association between the five-minute apgar score and neurodevelopmental outcomes among children aged 8−66 months in Australia. Int. J. Environ. Res. Public Health 2021, 18, 6450. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, T.; Lei, X.; Zhang, H.; Mao, M.; Zhang, J. The Apgar Score and Infant Mortality. PLoS ONE 2013, 8, e69072. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chauhan, S.P. Apgar score at 10 minutes and adverse outcomes among low-risk pregnancies. J. Matern. Fetal Neonatal Med. 2022, 35, 7109–7118. [Google Scholar] [CrossRef] [PubMed]
- Harron, K.; Gilbert, R.; Fagg, J.; Guttmann, A.; Van Der Meulen, J. Associations between pre-pregnancy psychosocial risk factors and infant outcomes: A population-based cohort study in England. Lancet Public Health 2021, 6, e97–e105. [Google Scholar] [CrossRef]
- Abadiga, M.; Mosisa, G.; Tsegaye, R.; Oluma, A.; Abdisa, E.; Bekele, T. Determinants of adverse birth outcomes among women delivered in public hospitals of Ethiopia, 2020. Arch. Public Health 2022, 80, 12. [Google Scholar] [CrossRef] [PubMed]
- Alhusen, J.L.; Bower, K.M.; Epstein, E.; Sharps, P. Racial Discrimination and Adverse Birth Outcomes: An Integrative Review. J. Midwifery Women’s Health 2016, 61, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Behrman, R.E.; Butler, A.S. Preterm Birth: Causes, Consequences, and Prevention; National Academies Press: Washington, DC, USA, 2007; ISBN 030910159X. [Google Scholar]
- Pedersen, M.; Giorgis-Allemand, L.; Bernard, C.; Aguilera, I.; Andersen, A.M.N.; Ballester, F.; Beelen, R.M.J.; Chatzi, L.; Cirach, M.; Danileviciute, A.; et al. Ambient air pollution and low birthweight: A European cohort study (ESCAPE). Lancet Respir. Med. 2013, 1, 695–704. [Google Scholar] [CrossRef]
- Mendola, P.; Nobles, C.; Williams, A.; Sherman, S.; Kanner, J.; Seeni, I.; Grantz, K. Air pollution and preterm birth: Do air pollution changes over time influence risk in consecutive pregnancies among low-risk women? Int. J. Environ. Res. Public Health 2019, 16, 3365. [Google Scholar] [CrossRef]
- Larsen, A.E.; Gaines, S.D.; Deschênes, O. Agricultural pesticide use and adverse birth outcomes in the San Joaquin Valley of California. Nat. Commun. 2017, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Wai, K.M.; Mar, O.; Kosaka, S.; Umemura, M.; Watanabe, C. Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study. Int. J. Environ. Res. Public Health 2017, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.; Kohn, E.; Daniel, S.; Hazan, A.; Berkovitch, M.; Brik, A.; Hochwald, O.; Borenstein-Levin, L.; Betser, M.; Moskovich, M.; et al. Prenatal exposure to heavy metal mixtures and anthropometric birth outcomes: A cross-sectional study. Environ. Health 2022, 21, 139. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Shinwari, N.; Mashhour, A.; Rabah, A. Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int. J. Hyg. Environ. Health 2014, 217, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Vigeh, M.; Nishioka, E.; Ohtani, K.; Omori, Y.; Matsukawa, T.; Koda, S.; Yokoyama, K. Prenatal mercury exposure and birth weight. Reprod. Toxicol. 2018, 76, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Irwinda, R.; Wibowo, N.; Putri, A.S. The Concentration of Micronutrients and Heavy Metals in Maternal Serum, Placenta, and Cord Blood: A Cross-Sectional Study in Preterm Birth. J. Pregnancy 2019, 2019, 5062365. [Google Scholar] [CrossRef] [PubMed]
- Bharti, J.; Vatsa, R.; Singhal, S.; Roy, K.K.; Kumar, S.; Perumal, V.; Meena, J. Pregnancy with chronic kidney disease: Maternal and fetal outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 204, 83–87. [Google Scholar] [CrossRef]
- Zhuang, X.; Cui, A.-M.; Wang, Q.; Cheng, X.-Y.; Shen, Y.; Cai, W.-H.; Li, H.-B.; Zhang, S.; Qin, G. Liver Dysfunction during Pregnancy and Its Association With Preterm Birth in China: A Prospective Cohort Study. eBioMedicine 2017, 26, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.T.; Almashhrawi, A.A.; Rahman, R.N.; Hammoud, G.M.; Ibdah, J.A. Liver diseases in pregnancy: Diseases unique to pregnancy. World J. Gastroenterol. 2013, 19, 7639–7646. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cabiddu, G.; Attini, R.; Vigotti, F.N.; Maxia, S.; Lepori, N.; Tuveri, M.; Massidda, M.; Marchi, C.; Mura, S.; et al. Risk of Adverse Pregnancy Outcomes in Women with CKD. J. Am. Soc. Nephrol. 2015, 26, 2011–2022. [Google Scholar] [CrossRef]
- Kendrick, J.; Sharma, S.; Holmen, J.; Palit, S.; Nuccio, E.; Chonchol, M. Kidney Disease and Maternal and Fetal Outcomes in Pregnancy. Am. J. Kidney Dis. 2015, 66, 55–59. [Google Scholar] [CrossRef]
- Nuyts, G.D.; Phd, H.; Elseviers, M.M.; De Broe, M.E. New occupational risk factors for chronic renal failure. Lancet 1995, 346, 7–11. [Google Scholar] [CrossRef]
- Choi, J.; Bae, S.; Lim, H.; Lim, J.-A.; Lee, Y.-H.; Ha, M.; Kwon, H.-J. Mercury Exposure in Association With Decrease of Liver Function in Adults: A Longitudinal Study. J. Prev. Med. Public Health 2017, 50, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Cave, M.; Appana, S.; Patel, M.; Falkner, K.C.; McClain, C.J.; Brock, G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ. Health Perspect. 2010, 118, 1735–1742. [Google Scholar] [CrossRef]
- Al-Neamy, F.R.M.; Almehdi, A.M.; Alwash, R.; Pasha, M.A.H.; Ibrahim, A.; Bener, A. Occupational lead exposure and amino acid profiles and liver function tests in industrial workers. Int. J. Environ. Health Res. 2001, 11, 960–3123. [Google Scholar] [CrossRef] [PubMed]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between exposure to heavy metals and the risk of chronic kidney disease: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Kort, S.; Wickliffe, J.K.; Shankar, A.; Shafer, M.; Hindori-Mohangoo, A.D.; Covert, H.H.; Lichtveld, M.Y.; Zijlmans, W. The Association between Mercury and Lead Exposure and Liver and Kidney Function in Pregnant Surinamese Women Enrolled in the Caribbean Consortium for Research in Environmental and Occupational Health (CCREOH) Environmental Epidemiologic Cohort Study Sheil. Toxics 2022, 10, 584. [Google Scholar] [CrossRef]
- Cardenas, A.; Roels, H.; Bernard, A.M.; Barbon, R.; Buchet, J.P.; Lauwerys, R.R.; Rosello, J.; Hotter, G.; Mutti, A.; Franchini, I.; et al. Markers of early renal changes induced by industrial pollutants. I. Application to workers exposed to mercury vapour. Br. J. Ind. Med. 1993, 50, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.E.; Bridges, C.C. Chronic kidney disease and exposure to nephrotoxic metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef]
- Verschueren, K.J.C.; Prüst, Z.D.; Paidin, R.R.; Kodan, L.R.; Bloemenkamp, K.W.M.; Rijken, M.J.; Browne, J.L. Childbirth outcomes and ethnic disparities in Suriname: A nationwide registry-based study in a middle-income country. Reprod. Health 2020, 17, 62. [Google Scholar] [CrossRef]
- WHO. Low Birth Weight, Prevalence (%). 2020. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/low-birth-weight-prevalence-(-) (accessed on 20 January 2024).
- Ouboter, P.E.; Landburg, G.; Satnarain, G.U.; Starke, S.Y.; Nanden, I.; Simon-Friedt, B.; Hawkins, W.B.; Taylor, R.; Lichtveld, M.Y.; Harville, E.; et al. Mercury levels in women and children from interior villages in Suriname, South America. Int. J. Environ. Res. Public Health 2018, 15, 1007. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile For Lead. 2007. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 11 April 2018).
- Carneiro, M.F.H.; Fabio, F.S.; Barbosa, F. Manioc flour consumption as a risk factor for lead poisoning in the Brazilian Amazon. J. Toxicol. Environ. Health-Part A Curr. Issues 2013, 76, 206–216. [Google Scholar] [CrossRef]
- Wickliffe, J.K.; Lichtveld, M.Y.; Zijlmans, C.W.; MacDonald-Ottevanger, S.; Shafer, M.; Dahman, C.; Harville, E.W.; Drury, S.; Landburg, G.; Ouboter, P.; et al. Exposure to total and methylmercury among pregnant women in Suriname: Sources and public health implications. J. Expo. Sci. Environ. Epidemiol. 2020, 31, 117–125. [Google Scholar] [CrossRef]
- Zijlmans, W.; Wickliffe, J.; Hindori-Mohangoo, A.; MacDonald-Ottevanger, S.; Ouboter, P.; Landburg, G.; Codrington, J.; Roosblad, J.; Baldewsingh, G.K.; Ramjatan, R.; et al. Caribbean Consortium for Research in Environmental and Occupational Health (CCREOH) Cohort Study: Influences of complex environmental exposures on maternal and child health in Suriname. BMJ Open 2020, 10, e034702. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liu, J.; Yuan, E.; Li, Y.; Wang, Q.; Jia, L.; Wang, L.; Su, Y. Gestational age-specific reference intervals for 15 biochemical measurands during normal pregnancy in China. Ann. Clin. Biochem. 2018, 55, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Carroll, R.J.; Wu, C.F.J.; Ruppert, D. The effect of estimating weights in weighted least squares. J. Am. Stat. Assoc. 1988, 83, 1045–1054. [Google Scholar] [CrossRef]
- WHO. WHO|Lead Poisoning and Health. Factsheet. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs379/en/ (accessed on 2 April 2018).
- Mahaffey, K.R.; Clickner, R.P.; Bodurow, C.C. Blood Organic Mercury and Dietary Mercury Intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ. Health Perspect. 2004, 112, 562–570. [Google Scholar] [CrossRef]
- Prüst, Z.D.; Kodan, L.R.; van den Akker, T.; Bloemenkamp, K.W.M.; Rijken, M.J.; Verschueren, K.J.C. The burden of severe hypertensive disorders of pregnancy on perinatal outcomes: A nationwide case-control study in Suriname. AJOG Glob. Rep. 2021, 1, 100027. [Google Scholar] [CrossRef]
- Adane, A.A.; Ayele, T.A.; Ararsa, L.G.; Bitew, B.D.; Zeleke, B.M. Adverse birth outcomes among deliveries at Gondar University Hospital, Northwest Ethiopia. BMC Pregnancy Childbirth 2014, 14, 90. [Google Scholar] [CrossRef]
- Lopes van Balen, V.A.; van Gansewinkel, T.A.G.; de Haas, S.; Spaan, J.J.; Ghossein-Doha, C.; van Kuijk, S.M.J.; van Drongelen, J.; Cornelis, T.; Spaanderman, M.E.A. Maternal kidney function during pregnancy: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2019, 54, 297–307. [Google Scholar] [CrossRef]
- Harville, E.W.; Catov, J.; Lewis, C.E.; Bibbins-Domingo, K.; Gunderson, E.P. Pre-pregnancy kidney function and subsequent adverse pregnancy outcomes. Pregnancy Hypertens. 2019, 15, 195–200. [Google Scholar] [CrossRef]
- Babay, Z.; Wakeel, J.A.; Addar, M.; Mittwalli, A.; Tarif, N.; Hammad, D.; Ali, N.; Al Askar, A.; Choudhary, A.R. Serum cystatin C in pregnant women: Reference values, reliable and superior diagnostic accuracy. Clin. Exp. Obstet. Gynecol. 2005, 32, 175. [Google Scholar] [PubMed]
- Harel, Z.; McArthur, E.; Hladunewich, M.; Dirk, J.S.; Wald, R.; Garg, A.X.; Ray, J.G. Serum Creatinine Levels Before, During, and after Pregnancy. JAMA-J. Am. Med. Assoc. 2019, 321, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Nzioka Mutua, D.; Nyaga, E.; Njagi, M.; Orinda, G.; Mutua, D.N. Liver Function Tests in Normal Pregnant Women. J. Liver 2018, 7, 2. [Google Scholar] [CrossRef]
- Johnston, J.E.; Valentiner, E.; Maxson, P.; Miranda, M.L.; Fry, R.C. Maternal cadmium levels during pregnancy associated with lower birth weight in infants in a North Carolina cohort. PLoS ONE 2014, 9, e109661. [Google Scholar] [CrossRef]
- Kippler, M.; Hoque, A.M.W.; Raqib, R.; Öhrvik, H.; Ekström, E.C.; Vahter, M. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol. Lett. 2010, 192, 162–168. [Google Scholar] [CrossRef]
- Abass, K.; Koiranen, M.; Mazej, D.; Tratnik, J.S.; Horvat, M.; Hakkola, J.; Järvelin, M.R.; Rautio, A. Arsenic, cadmium, lead and mercury levels in blood of Finnish adults and their relation to diet, lifestyle habits and sociodemographic variables. Environ. Sci. Pollut. Res. 2017, 24, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- CDC. Biomonitoring Data Tables for Environmental Chemicals. 2024. Available online: https://www.cdc.gov/exposurereport/data_tables.html (accessed on 15 June 2024).
- Wilhelm, M.; Ewers, U.; Schulz, C. Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine. Int. J. Hyg. Environ. Health 2004, 207, 69–73. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, B.K. National estimates of blood lead, cadmium, and mercury levels in the Korean general adult population. Int. Arch. Occup. Environ. Health 2011, 84, 53–63. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Biomonitorin-Mercury. 2022. Available online: https://www.epa.gov/americaschildrenenvironment/biomonitoring-mercury (accessed on 15 June 2024).
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Filipec-Kanizaj, T.; Jakopcic, I.; Majurec, I.; Brncic-Fischer, A.; Sobocan, N.; Hrstic, I.; Stimac, T.; Stimac, D.; Milic, S. Liver Disease During Pregnancy: A Challenging Clinical Issue. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 40820. [Google Scholar] [CrossRef]
- Shih, Y.H.; Chen, H.Y.; Christensen, K.; Handler, A.; Turyk, M.E.; Argos, M. Prenatal exposure to multiple metals and birth outcomes: An observational study within the National Children’s Study Cohort. Environ. Int. 2021, 147, 106373. [Google Scholar] [CrossRef]
- Thomas, S.; Arbuckle, T.E.; Fisher, M.; Fraser, W.D.; Ettinger, A.; King, W. Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: The MIREC study. Environ. Res. 2015, 140, 430–439. [Google Scholar] [CrossRef]
- Habibian Sezavar, A.; Abyareh, M.; Fahimi, R.; Nyasulu, P.S.; Abyadeh, M. The association between maternal cadmium exposure and small for gestational age: A systematic review and meta-analysis. Int. J. Environ. Health Res. 2022, 32, 1469–1477. [Google Scholar] [CrossRef]
- Huang, C.Y.; Liu, C.L.; Chen, M.H.; Tsao, P.N.; Chen, C.Y.; Chou, H.C.; Chen, P.C. Maternal exposure to air pollution and the risk of small for gestational age in offspring: A population-based study in Taiwan. Pediatr. Neonatol. 2020, 61, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Al-Rouqi, R.; Obsum, C.A.; Shinwari, N.; Mashhour, A.; Billedo, G.; Al-Sarraj, Y.; Rabbah, A. Mercury (Hg) and oxidative stress status in healthy mothers and its effect on birth anthropometric measures. Int. J. Hyg. Environ. Health 2014, 217, 567–585. [Google Scholar] [CrossRef] [PubMed]
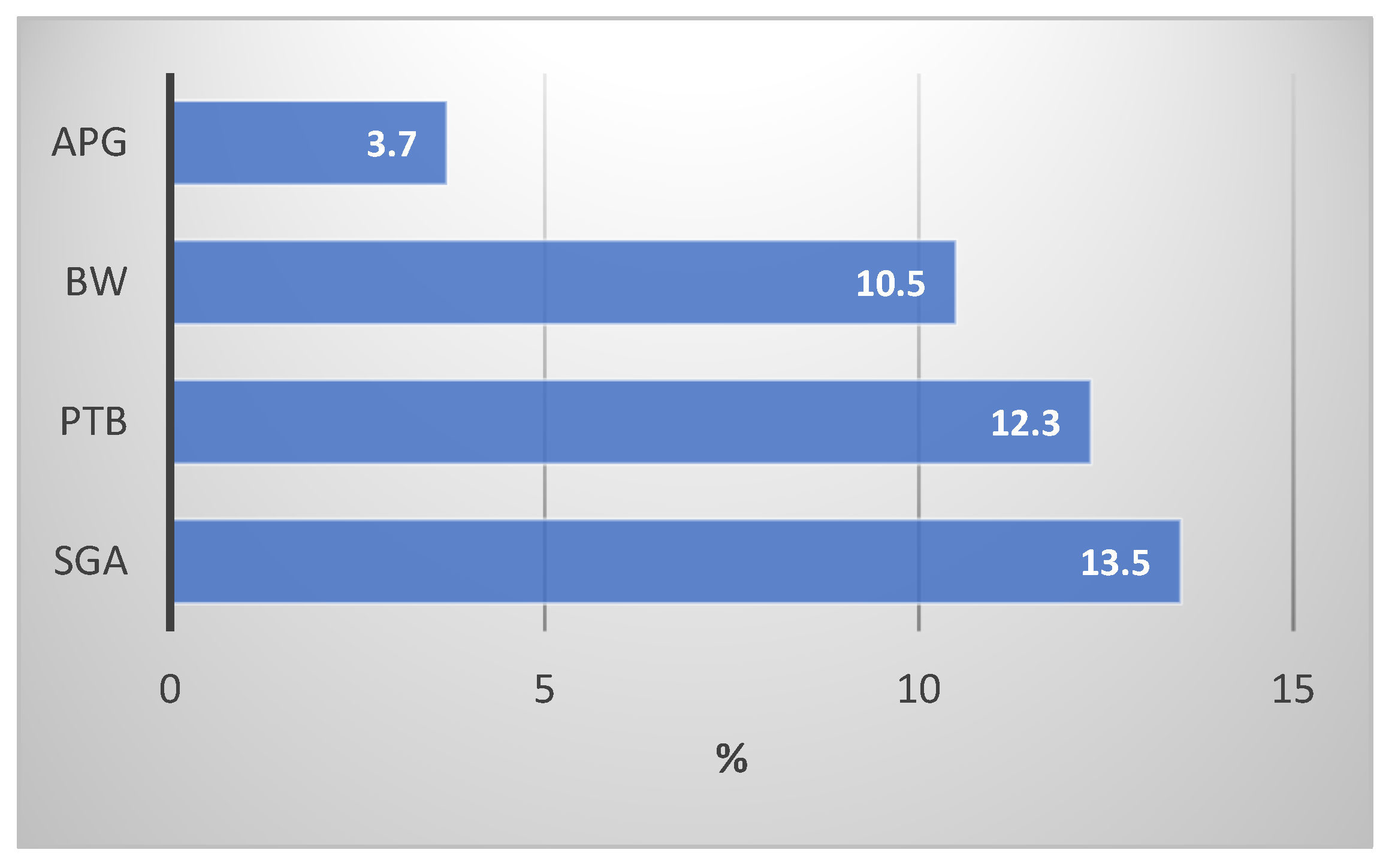
| (n = 400) | ||
|---|---|---|
| Paramater | Category | n (%) |
| Region | Paramaribo, Wanica, Commewijne, Saramacca, Para | 298 (74.5) |
| Nickerie, Coronie | 55 (13.8) | |
| Marowijne, Brokopondo, Sipaliwini | 47 (11.8) | |
| Maternal age at intake | 16–19 years | 40 (10.0) |
| 20–34 years | 301 (75.3) | |
| 35+ years | 59 (14.8) | |
| Ethnicity * | African descent | 187 (46.8) |
| Asian descent | 102 (25.5) | |
| Other | 110 (27.5) | |
| Education | No or primary | 72 (18.0) |
| Lower vocational or secondary | 148 (37.0) | |
| Upper secondary/vocational or tertiary | 179 (44.8) |
| Biomarker/Element | Median | IQR | Reference or Normal Concentration |
|---|---|---|---|
| Creat (umol/L) | 49.00 | 13 | 44–97 |
| Ur (mmol/L) | 2.50 | 0.7 | 1.8–7.1 |
| CysC (µg/mL) | 0.62 | 0.17 | 0.57–1.79 |
| AST (U/L) | 12.00 | 5 | 15–41 |
| ALT (U/L) | 9.00 | 3 | 7–52 |
| GGT (U/L) | 11.00 | 8 | 7–50 |
| Bl Al (µg/L) | 11.90 | 10.74 | 1–3 |
| Bl Mn (µg/L) | 15.14 | 9.78 | 9.5 |
| Bl Fe (µg/dL) | 395,520.34 | 92,941.74 | 50–212 |
| Bl Se (µg/L) | 193.02 | 46.89 | 125–163 |
| Bl Cd (µg/L) | 0.26 | 0.18 | 0.315 |
| Bl Sn (µg/L) | 0.67 | 0.63 | # |
| Bl Hg (µg/L) | 2.86 | 2.72 | 0.6–0.9 |
| Bl Pb (µg/dL) | 1.98 | 1.94 | ≥5 * |
| Gestational Age | Birthweight | Apgar Score at 5 min | |||||
|---|---|---|---|---|---|---|---|
| Parameter | Beta | p-Value | Beta | p-Value | Beta | p-Value | |
| Kidney | Blood Cr | 0.009 | 0.522 | 0.002 | 0.441 | <0.0001 | 0.937 |
| Blood Ur | 0.131 | 0.569 | −0.04 | 0.220 | 0.427 | 0.002 | |
| Blood CysC | 1.412 | 0.006 | 0.072 | 0.381 | −0.640 | <0.0001 * | |
| Liver | Blood AST | −0.111 | 0.0004 * | 0.004 | 0.299 | −0.060 | 0.004 * |
| Blood ALT | 0.181 | <0.0001 * | >−0.001 | 0.909 | 0.0412 | 0.044 | |
| Blood GGT | 0.028 | 0.142 | −0.001 | 0.668 | >−0.0001 | 0.975 | |
| Blood Al | <0.001 | 0.99 | <0.0001 | 0.039 * | −0.0169 | 0.004 * | |
| Metals | Blood Mn | 0.051 | <0.0001 * | −0.001 | 0.677 | −0.007 | 0.398 |
| Blood Fe | <0.001 | 0.0001 * | −<0.001 | 0.923 | >−0.001 | <0.0001 * | |
| Blood Se | −0.025 | <0.0001 * | >−0.001 | 0.554 | 0.003 | 0.057 | |
| Blood Cd | 5.454 | <0.0001 * | −0.050 | 0.721 | 0.464 | 0.263 | |
| Blood Sn | 0.010 | 0.632 | 0.006 | 0.222 | −0.006 | 0.631 | |
| Blood Hg | −0.027 | 0.515 | 0.007 | 0.222 | −0.025 | 0.076 | |
| Blood Pb | 0.049 | 0.197 | −0.011 | 0.166 | 0.020 | 0.354 | |
| Region # | Rural | 0.031 | 0.906 | −0.201 | 0.0006 * | −1.033 | <0.0001 * |
| Interior | 2.131 | <0.0001 * | 0.078 | 0.229 | −0.030 | 0.853 | |
| Urban | 0 | 0 | 0 | ||||
| Maternal Age | 16-19 | −1.338 | <0.0001 * | −0.108 | 0.052 | 0.666 | 0.001 * |
| 35+ | −1.283 | 0.0005 * | 0.004 | 0.946 | −0.231 | 0.143 | |
| 20–34 | 0 | 0 | 0 | ||||
| Ethnicity | Asian | −0.255 | 0.513 | 0.132 | 0.024 | 0.021 | 0.920 |
| Else | 0.664 | 0.006 | 0.072 | 0.099 | 0.254 | 0.087 | |
| African | 0 | 0 | 0 | ||||
| Education | Secondary | −0.194 | 0.616 | −0.031 | 0.606 | −0.477 | 0.0003 * |
| Tertriary | 0.243 | 0.589 | −0.029 | 0.689 | 0.074 | 0.722 | |
| No/primary | 0 | 0 | 0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kort, S.; Wickliffe, J.; Shankar, A.; Covert, H.H.; Lichtveld, M.; Zijlmans, W. Association between Liver and Kidney Function and Birth Outcomes in Pregnant Surinamese Women Exposed to Mercury and Lead in the Caribbean Consortium for Research in Environmental and Occupational Health (CCREOH) Environmental Epidemiologic Cohort Study. J. Xenobiot. 2024, 14, 1051-1063. https://doi.org/10.3390/jox14030059
Kort S, Wickliffe J, Shankar A, Covert HH, Lichtveld M, Zijlmans W. Association between Liver and Kidney Function and Birth Outcomes in Pregnant Surinamese Women Exposed to Mercury and Lead in the Caribbean Consortium for Research in Environmental and Occupational Health (CCREOH) Environmental Epidemiologic Cohort Study. Journal of Xenobiotics. 2024; 14(3):1051-1063. https://doi.org/10.3390/jox14030059
Chicago/Turabian StyleKort, Sheila, Jeffrey Wickliffe, Arti Shankar, Hannah H. Covert, Maureen Lichtveld, and Wilco Zijlmans. 2024. "Association between Liver and Kidney Function and Birth Outcomes in Pregnant Surinamese Women Exposed to Mercury and Lead in the Caribbean Consortium for Research in Environmental and Occupational Health (CCREOH) Environmental Epidemiologic Cohort Study" Journal of Xenobiotics 14, no. 3: 1051-1063. https://doi.org/10.3390/jox14030059
APA StyleKort, S., Wickliffe, J., Shankar, A., Covert, H. H., Lichtveld, M., & Zijlmans, W. (2024). Association between Liver and Kidney Function and Birth Outcomes in Pregnant Surinamese Women Exposed to Mercury and Lead in the Caribbean Consortium for Research in Environmental and Occupational Health (CCREOH) Environmental Epidemiologic Cohort Study. Journal of Xenobiotics, 14(3), 1051-1063. https://doi.org/10.3390/jox14030059









