Evaluation of Cytotoxic and Genotoxic Effects in Buccal Mucosal Cells in Non-Smokers and Users of Traditional Combustible Tobacco Products and Non-Combustible Alternatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cell Sampling
2.3. Statistical Analysis
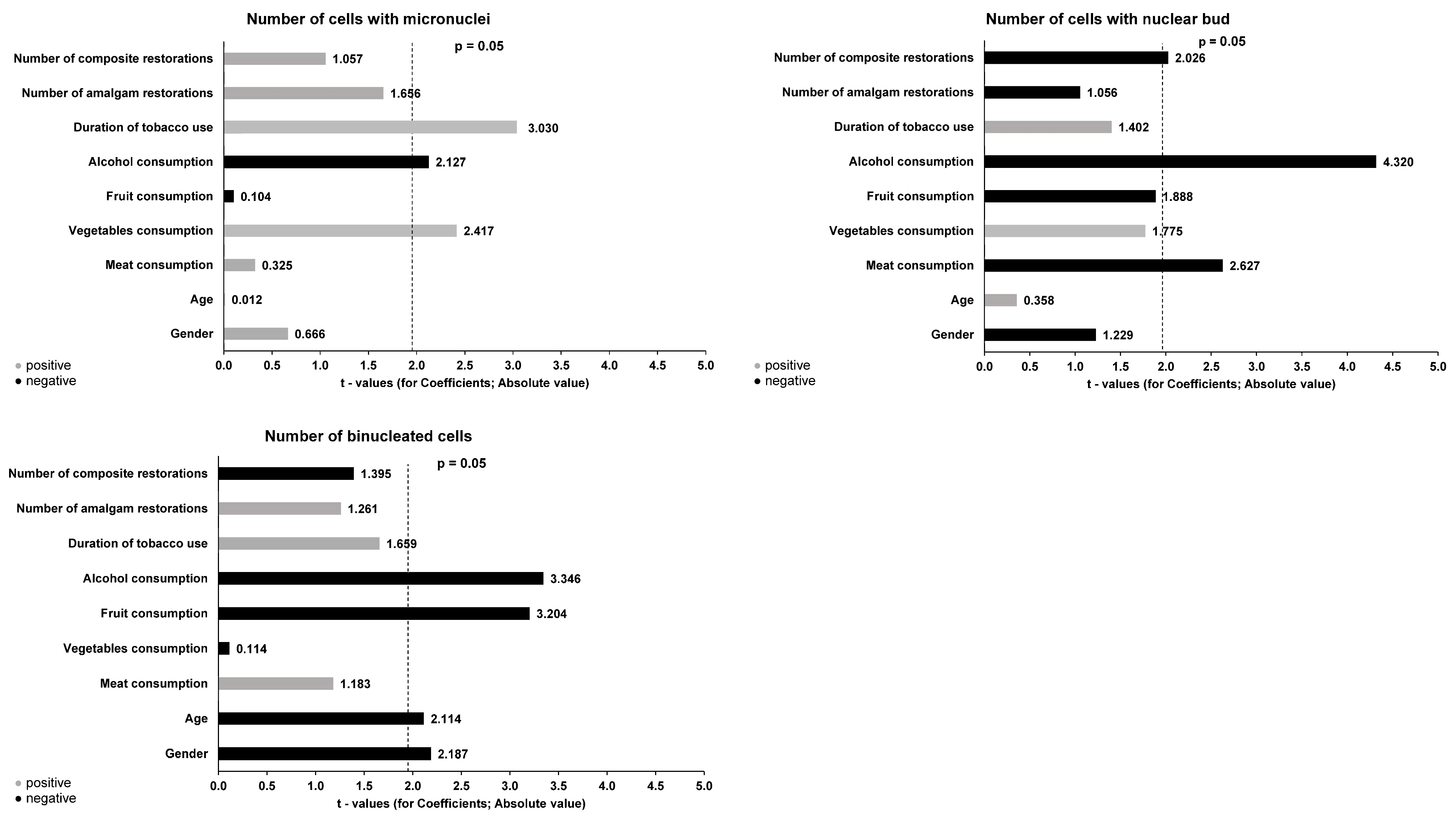
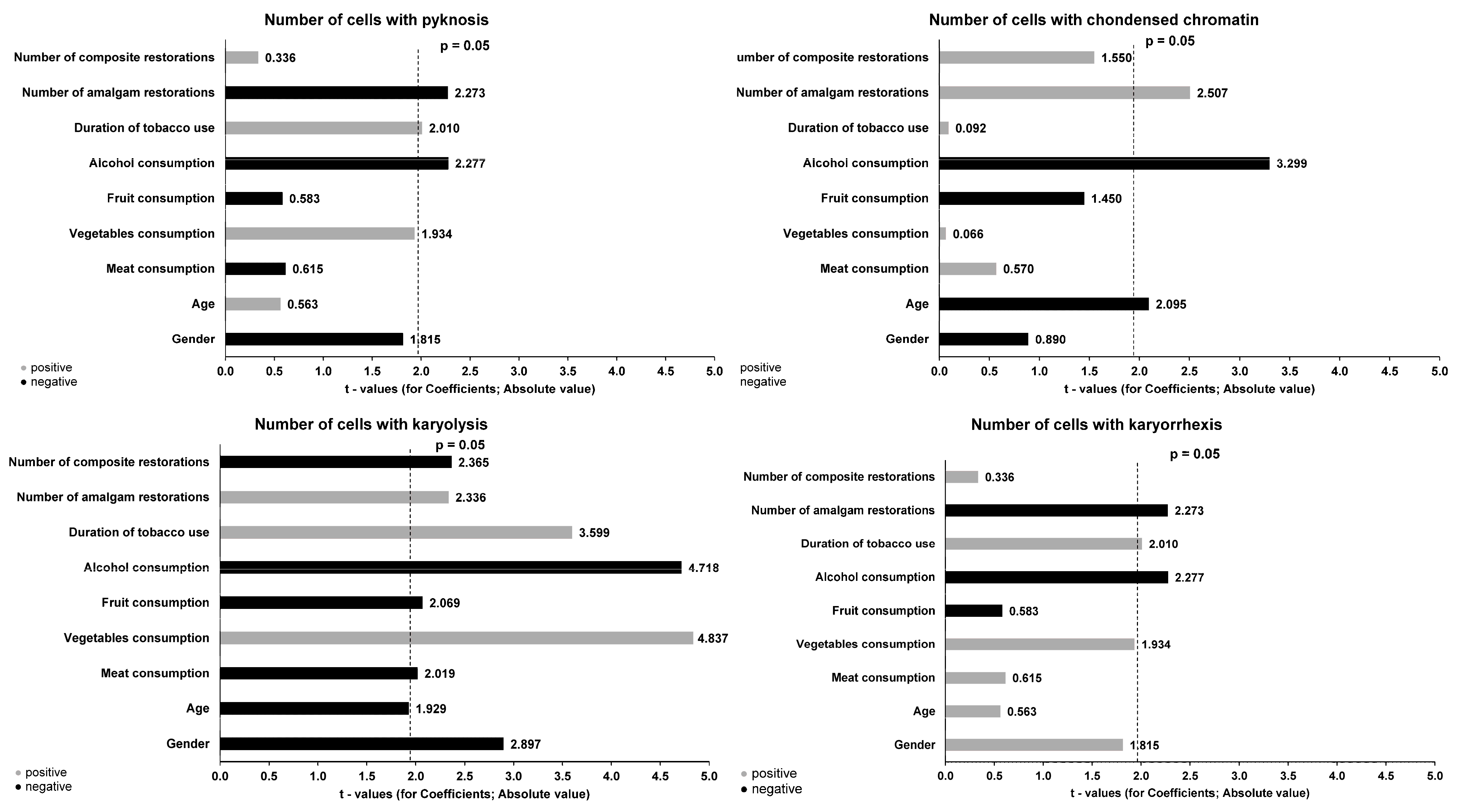
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- West, R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 1018–1036. [Google Scholar] [CrossRef]
- Vukas, J.; Mallock-Ohnesorg, N.; Ruther, T.; Pieper, E.; Romano-Brandt, L.; Stoll, Y.; Hoehne, L.; Burgmann, N.; Laux, P.; Luch, A.; et al. Two Different Heated Tobacco Products vs. Cigarettes: Comparison of Nicotine Delivery and Subjective Effects in Experienced Users. Toxics 2023, 11, 525. [Google Scholar] [CrossRef]
- Soleimani, F.; Dobaradaran, S.; De-la-Torre, G.E.; Schmidt, T.C.; Saeedi, R. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: A comprehensive systematic review. Sci. Total. Environ. 2022, 813, 152667. [Google Scholar] [CrossRef] [PubMed]
- Giovacchini, C.X.; Crotty, A.L.E.; Que, L.G. Electronic Cigarettes: A Pro-Con Review of the Current Literature. J. Allergy Clin. Immunol. Pract. 2022, 10, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Karey, E.; Rebuli, M.E.; Escobar, Y.N.H.; Jaspers, I.; Chen, L.C. E-Cigarette Toxicology. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Simonavicius, E.; McNeill, A.; Shahab, L.; Brose, L.S. Heat-not-burn tobacco products: A systematic literature review. Tob. Control. 2019, 28, 582–594. [Google Scholar] [CrossRef]
- Upadhyay, S.; Rahman, M.; Johanson, G.; Palmberg, L.; Ganguly, K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics 2023, 11, 667. [Google Scholar] [CrossRef]
- Schaller, J.P.; Keller, D.; Poget, L.; Pratte, P.; Kaelin, E.; McHugh, D.; Cudazzo, G.; Smart, D.; Tricker, A.R.; Gautier, L.; et al. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016, 81, S27–S47. [Google Scholar] [CrossRef]
- Thorne, D.; Leverette, R.; Breheny, D.; Lloyd, M.; McEnaney, S.; Whitwell, J.; Clements, J.; Bombick, B.; Gaça, M. Genotoxicity evaluation of tobacco and nicotine delivery products: Part Two. In vitro micronucleus assay. Food Chem. Toxicol. 2019, 132, 110546. [Google Scholar] [CrossRef]
- Thorne, D.; Whitwell, J.; Clements, J.; Walker, P.; Breheny, D.; Gaca, M. The genotoxicological assessment of a tobacco heating product relative to cigarette smoke using the in vitro micronucleus assay. Toxicol. Rep. 2020, 7, 1010–1019. [Google Scholar] [CrossRef]
- Boyle, J.O.; Gumus, Z.H.; Kacker, A.; Choksi, V.L.; Bocker, J.M.; Zhou, X.K.; Yantiss, R.K.; Hughes, D.B.; Du, B.; Judson, B.L.; et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev. Res. 2020, 3, 266–278. [Google Scholar] [CrossRef]
- Alharbi, I.A.; Rouabhia, M. Repeated exposure to whole cigarette smoke promotes primary human gingival epithelial cell growth and modulates keratin expression. J. Periodontal. Res. 2016, 51, 630–638. [Google Scholar] [CrossRef]
- Abbott, A.J.; Reibel, Y.G.; Arnett, M.C.; Marka, N.; Drake, M.A. Oral and Systemic Health Implications of Electronic Cigarette Usage as Compared to Conventional Tobacco Cigarettes: A review of the literature. J. Dent. Hyg. 2023, 97, 21–35. [Google Scholar] [PubMed]
- Hamann, S.L.; Kungskulniti, N.; Charoenca, N.; Kasemsup, V.; Ruangkanchanasetr, S.; Jongkhajornpong, P. Electronic Cigarette Harms: Aggregate Evidence Shows Damage to Biological Systems. Int. J. Environ. Res. Public Health 2023, 20, 6808. [Google Scholar] [CrossRef] [PubMed]
- Znyk, M.; Jurewicz, J.; Kaleta, D. Exposure to Heated Tobacco Products and Adverse Health Effects, a Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6651. [Google Scholar] [CrossRef] [PubMed]
- Goebel, I.; Mohr, T.; Axt, P.N.; Watz, H.; Trinkmann, F.; Weckmann, M.; Drömann, D.; Franzen, K.F. Impact of Heated Tobacco Products, E-Cigarettes, and Combustible Cigarettes on Small Airways and Arterial Stiffness. Toxics 2023, 11, 758. [Google Scholar] [CrossRef]
- Nersesyan, A.; Muradyan, R.; Kundi, M.; Knasmueller, S. Impact of smoking on the frequencies of micronuclei and other nuclear abnormalities in exfoliated oral cells: A comparative study with different cigarette types. Mutagenesis 2011, 26, 295–301. [Google Scholar] [CrossRef]
- Naderi, N.J.; Farhadi, S.; Sarshar, S. Micronucleus assay of buccal mucosa cells in smokers with the history of smoking less and more than 10 years. Indian. J. Pathol. Microbiol. 2012, 55, 433–438. [Google Scholar] [CrossRef]
- Haveric, A.; Haveric, S.; Ibrulj, S. Micronuclei frequencies in peripheral blood and buccal exfoliated cells of young smokers and non-smokers. Toxicol. Mech. Methods 2010, 20, 260–266. [Google Scholar] [CrossRef]
- Pop, A.M.; Coros, R.; Stoica, A.M.; Monea, M. Early Diagnosis of Oral Mucosal Alterations in Smokers and E-Cigarette Users Based on Micronuclei Count: A Cross-Sectional Study among Dental Students. Int. J. Environ. Res. Public Health 2012, 18, 6246. [Google Scholar] [CrossRef]
- Dash, K.C.; Nishat, R.; Kumar, H.; Mishra, S.; Raghuvanshi, M.; Bajoria, A. Comparative Study of Micronuclei Count in Patients with Different Tobacco-related Habits using Exfoliated Buccal Epithelial Cells: A Tool for Assessment of Genotoxicity. J. Contemp. Dent. Pract. 2018, 19, 1076–1081. [Google Scholar] [PubMed]
- Schwarzmeier, L.A.T.; da Cruz, B.S.; Ferreira, C.C.P.; Carvalho, B.; Alves, M.G.O.; Lima Carta, C.F.; Scholz, J.R.; Almeida, J.D. E-cig might cause cell damage of oral mucosa. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2021, 131, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Zarcone, G.; Lenski, M.; Martinez, T.; Talahari, S.; Simonin, O.; Garcon, G.; Allorge, D.; Nesslany, F.; Lo-Guidice, J.-M.; Platel, A.; et al. Impact of Electronic Cigarettes, Heated Tobacco Products and Conventional Cigarettes on the Generation of Oxidative Stress and Genetic and Epigenetic Lesions in Human Bronchial Epithelial BEAS-2B Cells. Toxics 2023, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Elkhatib, R.; Al-Rajoudi, T.; Al-Qudaihi, G.; Manogarannogaran, P.; Eltabache, C.; Alotaibi, A.; Bin Mummer, A.; Almugbel, S. Cytotoxic and genotoxic effects of e-liquids and their potential associations with nicotine, menthol and phthalate esters. Chemosphere 2020, 249, 126153. [Google Scholar] [CrossRef]
- Misra, M.; Leverette, R.D.; Cooper, B.T.; Bennett, M.B.; Brown, S.E. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: E-liquids, extracts and collected aerosols. Int. J. Environ. Res. Public Health 2014, 11, 11325–11347. [Google Scholar] [CrossRef]
- Bolognesi, C.; Bonassi, S.; Knasmueller, S.; Fenech, M.; Bruzzone, M.; Lando, C.; Ceppi, M. Clinical application of micronucleus test in exfoliated buccal cells: A systematic review and metanalysis. Mutat. Res. Rev. Mutat. Res. 2015, 766, 20–31. [Google Scholar] [CrossRef]
- Upadhyay, M.; Verma, P.; Sabharwal, R.; Subudhi, S.K.; Jatol-Tekade, S.; Naphade, V.; Choudhury, B.K.; Sahoo, P.D. Micronuclei in Exfoliated Cells: A Biomarker of Genotoxicity in Tobacco Users. Niger. J. Surg. 2019, 25, 52–59. [Google Scholar] [CrossRef]
- Thomas, P.; Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. Buccal micronucleus cytome assay. Nat. Protoc. 2009, 4, 825–837. [Google Scholar] [CrossRef]
- Tolbert, P.E.; Shy, C.M.; Allen, J.W. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat. Res. 1992, 271, 69–77. [Google Scholar] [CrossRef]
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef] [PubMed]
- DeMarini, D.M. Genotoxicity of tobacco smoke and tobacco smoke condensate: A review. Mutat. Res. 2004, 567, 447–474. [Google Scholar] [CrossRef]
- Chaffee, B.W.; Couch, E.T.; Vora, M.V.; Holliday, R.S. Oral and periodontal implications of tobacco and nicotine products. Periodontology 2000 2021, 87, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, F.; Sewer, A.; Scotti, E.; Titz, B.; Schlage, W.K.; Leroy, P.; Kondylis, A.; Vuillaume, G.; Iskandar, A.R.; Guedj, E.; et al. Assessment of the impact of aerosol from a potential modified risk tobacco product compared with cigarette smoke on human organotypic oral epithelial cultures under different exposure regimens. Food Chem. Toxicol. 2018, 115, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Torres-Bugarin, O.; Zavala-Cerna, M.G.; Nava, A.; Flores-Garcia, A.; Ramos-Ibarra, M.L. Potential uses, limitations, and basic procedures of micronuclei and nuclear abnormalities in buccal cells. Dis. Markers 2014, 2014, 956835. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.A.; Loh, C.H.; Hsieh, L.L.; Liu, T.Y.; Chen, C.J.; Liou, S.H. Clastogenic effect for cigarette smoking but not areca quid chewing as measured by micronuclei in exfoliated buccal mucosal cells. Mutat. Res. 2004, 562, 27–38. [Google Scholar] [CrossRef]
- Bansal, H.; Sandhu, V.S.; Bhandari, R.; Sharma, D. Evaluation of micronuclei in tobacco users: A study in Punjabi population. Contemp. Clin. Dent. 2012, 3, 184–187. [Google Scholar] [CrossRef]
- Ozkul, Y.; Donmez, H.; Erenmemisoglu, A.; Demirtas, H.; Imamoglu, N. Induction of micronuclei by smokeless tobacco on buccal mucosa cells of habitual users. Mutagenesis 1997, 12, 285–287. [Google Scholar] [CrossRef]
- Farhadi, S.; Mohamadi, M.; Mohamadi, M. Repair Index in Examination of Nuclear Changes in the Buccal Mucosa of Smokers: A Useful Method for Screening of Oral Cancer. Asian. Pac. J. Cancer Prev. 2017, 18, 3087–3090. [Google Scholar] [CrossRef]
- Kamath, V.; Anigol, P.; Setlur, K. Micronuclei as prognostic indicators in oral cytological smears: A comparison between smokers and non-smokers. Clin. Cancer Investig. J. 2014, 3, 49–54. [Google Scholar] [CrossRef]
- Burgaz, S.; Iscan, A.; Buyukbingol, Z.K.; Bozkurt, A.; Karakaya, A.E. Evaluation of micronuclei in exfoliated urothelial cells and urinary thioether excretion of smokers. Mutat. Res. 1995, 335, 163–169. [Google Scholar] [CrossRef]
- Nersesyan, A.; Muradyan, R.; Kundi, M.; Fenech, M.; Bolognesi, C.; Knasmueller, S. Smoking causes induction of micronuclei and other nuclear anomalies in cervical cells. Int. J. Hyg. Environ. Health 2020, 226, 113492. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, H.A.; Karim, K.K.; Othman, G.O.; Mohammed, A.A.; Salih, A.M.; Zuhdi, S.S.; Jamel, D. DNA damage level in lymphocytes and buccal epithelial cells in Narghile smokers and non-smokers in Duhok city-KRG-Iraq. Oral Oncol. Rep. 2022, 1–2, 100002. [Google Scholar] [CrossRef]
- Yu, V.; Rahimy, M.; Korrapati, A.; Xuan, Y.; Zou, A.E.; Krishnan, A.R.; Tsui, T.; Aguilera, J.A.; Advani, S.; Alexander, L.E.C.; et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral. Oncol. 2016, 52, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Franco, T.; Trapasso, S.; Puzzo, L.; Allegra, E. Electronic Cigarette: Role in the Primary Prevention of Oral Cavity Cancer. Clin. Med. Insights Ear Nose Throat 2016, 9, 7–12. [Google Scholar] [CrossRef]
- Ratajczak, A.; Jankowski, P.; Strus, P.; Feleszko, W. Heat Not Burn Tobacco Product-A New Global Trend: Impact of Heat-Not-Burn Tobacco Products on Public Health, a Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 409. [Google Scholar] [CrossRef]
- Majek, P.; Jankowski, M.; Brozek, G.M. Acute health effects of heated tobacco products: Comparative analysis with traditional cigarettes and electronic cigarettes in young adults. ERJ Open Res. 2023, 9, 00595-2022. [Google Scholar] [CrossRef] [PubMed]
- Bekki, K.; Inaba, Y.; Uchiyama, S.; Kunugita, N. Comparison of Chemicals in Mainstream Smoke in Heat-not-burn Tobacco and Combustion Cigarettes. J. UOEH 2017, 39, 201–207. [Google Scholar] [CrossRef]
- Bonassi, S.; Neri, M.; Lando, C.; Ceppi, M.; Lin, Y.P.; Chang, W.P.; Holland, N.; Kirsch-Volders, M.; Zeiger, E.; Fenech, M. Effect of smoking habit on the frequency of micronuclei in human lymphocytes: Results from the Human MicroNucleus project. Mutat. Res. 2003, 543, 155–166. [Google Scholar] [CrossRef]
- Gasche, J.A.; Goel, A. Epigenetic mechanisms in oral carcinogenesis. Future Oncol. 2012, 8, 1407–1425. [Google Scholar] [CrossRef]
| Characteristics | Non-Smokers (n = 40) | Cigarette (n = 40) | E-Cigarette (n = 40) | Tobacco Heating Product (n = 40) | Total (n = 160) | |
|---|---|---|---|---|---|---|
| Age | 47.8 ± 12.8 | 43.1 ± 16.3 | 40.1 ± 14.5 | 35.4 ± 12.2 | 41.6 ± 14.6 | |
| Gender | Male | 20 (50.0) | 12 (30.0) | 13 (32.5) | 16 (40.0) | 61 (38.2) |
| Female | 20 (50.0) | 28 (70.0) | 27 (67.5) | 24 (60.0) | 99 (61.8) | |
| Duration of product use (years) | 0 | 13.3 ± 13.9 | 5.1 ± 3.4 | 2.3 ± 0.7 | 6.9 ± 9.4 | |
| Cytogenetic Parameters | Non-Smokers | Cigarette | E-Cigarette | Tobacco Heating Product | p Values | |
|---|---|---|---|---|---|---|
| Number of cells with micronuclei | Mdn (IQR) | 1.00 (0.00−2.00) A | 2.00 (1.00−2.50) | 2.00 (1.25−2.00) A | 1.50 (0.00−2.00) | 0.011 * |
| M ± SD | 1.22 ± 1.00 | 1.95 ± 1.46 | 1.85 ± 0.58 | 1.40 ± 1.17 | ||
| Number of cells with nuclear bud | Mdn (IQR) | 0.00 (0.00−1.00) B,C | 1.00 (0.00−1.00) | 1.00 (0.00−1.00) B | 1.00 (0.00−1.50) C | 0.012 * |
| M ± SD | 0.43 ± 0.63 | 0.85 ± 0.80 | 0.90 ± 0.74 | 0.90 ± 0.81 | ||
| Number of binucleated cells | Mdn (IQR) | 5.00 (3.00−5.75) D,E,F | 7.00 (2.25−9.00) D | 7.00 (5.00−9.00) E | 7.00 (5.00−5.00) F | ≤0.001 * |
| M ± SD | 4.50 ± 2.44 | 6.37 ± 3.64 | 7.35 ± 2.45 | 7.30 ± 2.90 | ||
| Number of cells with karyorrhexis | Mdn (IQR) | 6.00 (4.25−7.00) G,H,I | 8.50 (6.00−11.00) G | 10.00 (8.00−12.00) H | 8.00 (7.00−11.00) I | ≤0.001 * |
| M ± SD | 6.25 ± 2.47 | 8.58 ± 3.17 | 9.98 ± 2.13 | 8.87 ± 3.24 | ||
| Number of cells with karyolysis | Mdn (IQR) | 9.50 (4.25−9.50) J,K,L | 16.00 (10.00−18.00) J | 15.00 (11.00−18.00) K | 15.00 (13.00− 17.00) L | ≤0.001 * |
| M ± SD | 8.73 ± 3.14 | 14.75 ± 5.56 | 14.85 ± 3.54 | 14.88 ± 3.86 | ||
| Number of cells with condensed chromatin | Mdn (IQR) | 4.00 (2.00−8.75) M,N | 8.00 (4.25−9.00) O | 10.00 (8.00−11.00) M,O | 8.50 (6.25−10.75) N | ≤0.001 * |
| M ± SD | 4.88 ± 3.73 | 7.12 ± 3.01 | 9.70 ± 1.63 | 8.80 ± 2.80 | ||
| Number of cells with pyknosis | Mdn (IQR) | 4.00 (4.00−6.00) P,Q | 5.00 (4.25−9.00) R | 6.00 (4.25−8.00) P | 7.50 (4.50−9.00) Q,R | ≤0.001 * |
| M ± SD | 4.35 ± 1.98 | 5.12 ± 1.80 | 6.24 ± 1.99 | 6.90 ± 2.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadin, A.; Stazic, V.; Galic, N.; Zeljezic, D. Evaluation of Cytotoxic and Genotoxic Effects in Buccal Mucosal Cells in Non-Smokers and Users of Traditional Combustible Tobacco Products and Non-Combustible Alternatives. J. Xenobiot. 2024, 14, 154-165. https://doi.org/10.3390/jox14010009
Tadin A, Stazic V, Galic N, Zeljezic D. Evaluation of Cytotoxic and Genotoxic Effects in Buccal Mucosal Cells in Non-Smokers and Users of Traditional Combustible Tobacco Products and Non-Combustible Alternatives. Journal of Xenobiotics. 2024; 14(1):154-165. https://doi.org/10.3390/jox14010009
Chicago/Turabian StyleTadin, Antonija, Vinka Stazic, Nada Galic, and Davor Zeljezic. 2024. "Evaluation of Cytotoxic and Genotoxic Effects in Buccal Mucosal Cells in Non-Smokers and Users of Traditional Combustible Tobacco Products and Non-Combustible Alternatives" Journal of Xenobiotics 14, no. 1: 154-165. https://doi.org/10.3390/jox14010009
APA StyleTadin, A., Stazic, V., Galic, N., & Zeljezic, D. (2024). Evaluation of Cytotoxic and Genotoxic Effects in Buccal Mucosal Cells in Non-Smokers and Users of Traditional Combustible Tobacco Products and Non-Combustible Alternatives. Journal of Xenobiotics, 14(1), 154-165. https://doi.org/10.3390/jox14010009






