Spironolactone Induces Vasodilation by Endothelium-Dependent Mechanisms Involving NO and by Endothelium-Independent Mechanisms Blocking Ca2+ Channels
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Considerations
2.2. Experimental Model
2.3. Isolation of Rat Aortic Rings
2.4. Ex Vivo Experiments
2.4.1. Vasorelaxant Effect of Spironolactone on Isolated Rat Aorta
2.4.2. Effects of Spironolactone-Induced Relaxation via L-Type Ca2+ Channels (LTCC)
2.4.3. Effects of Spironolactone-Induced Relaxation via Endothelium
2.5. Smooth Muscle Cell (SMC) Culture
2.6. SMC Cytotoxicity Assay
2.7. Drugs and Chemicals
2.8. Data and Statistical Analysis
3. Results
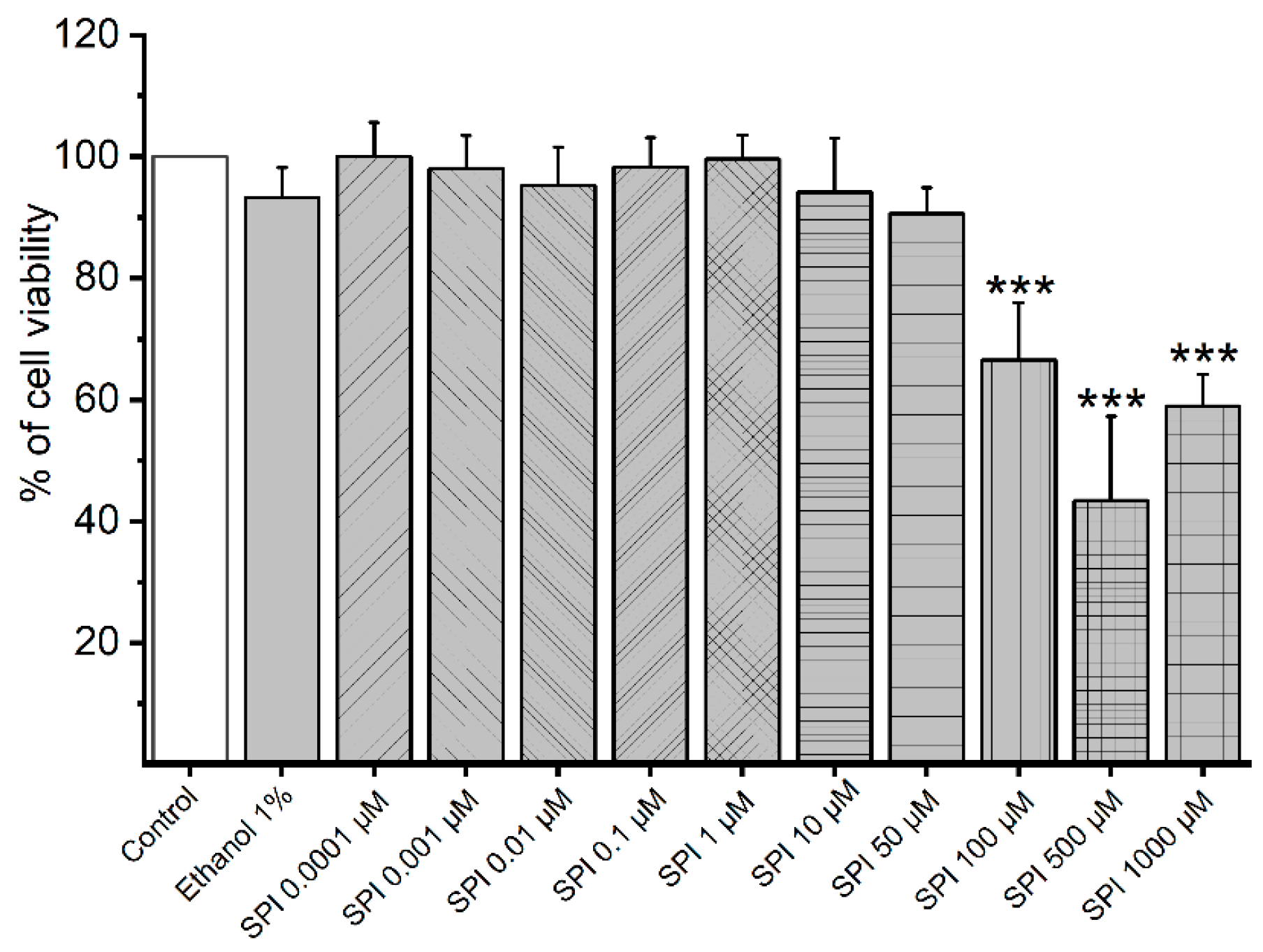
3.1. Cytotoxicity of Smooth Muscle Cells Induced by SPI
3.2. Contractility Experiments on Rat Aorta Smooth Muscle
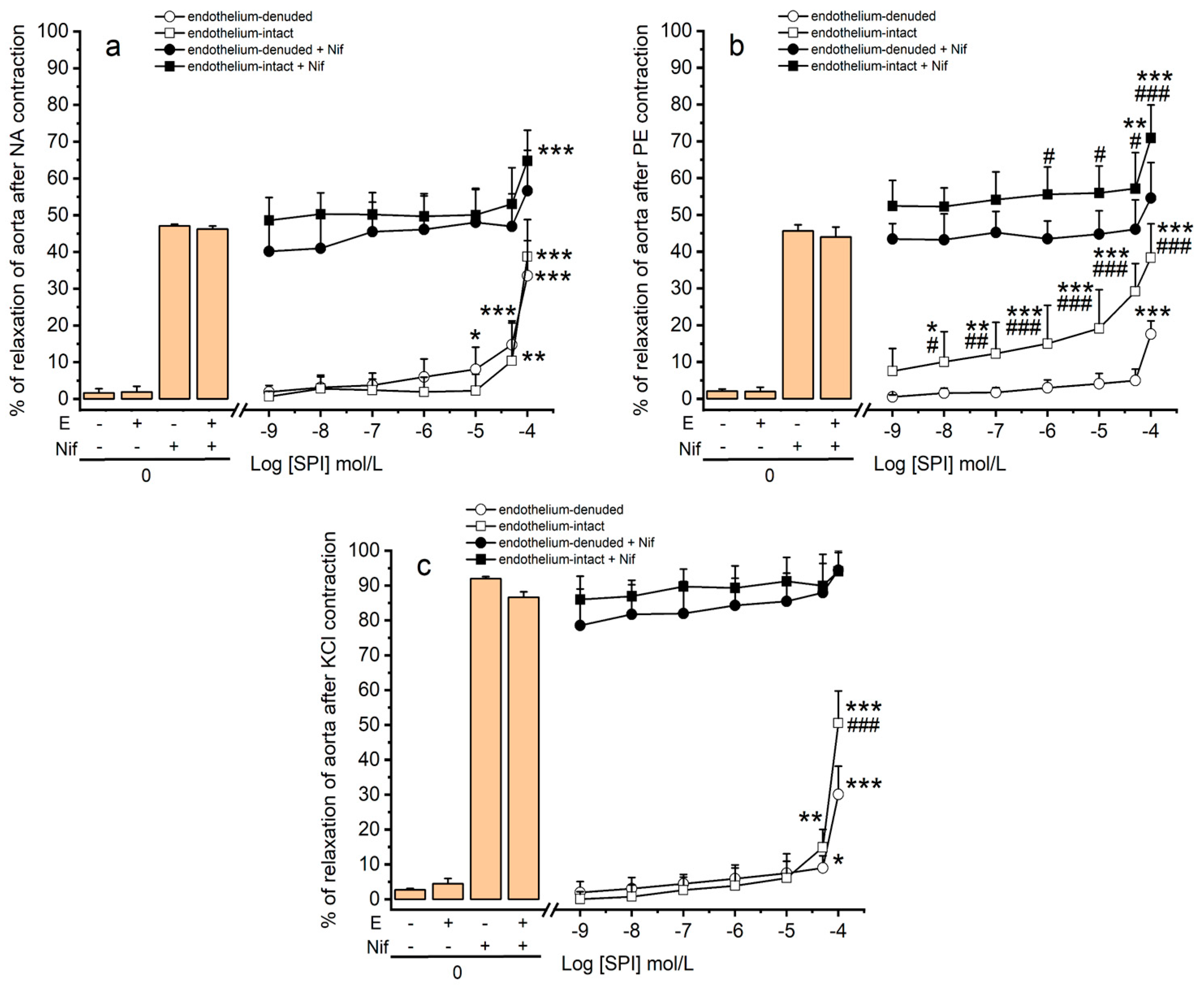
3.3. Vasodilation Induced by SPI through Ca2+ Channels
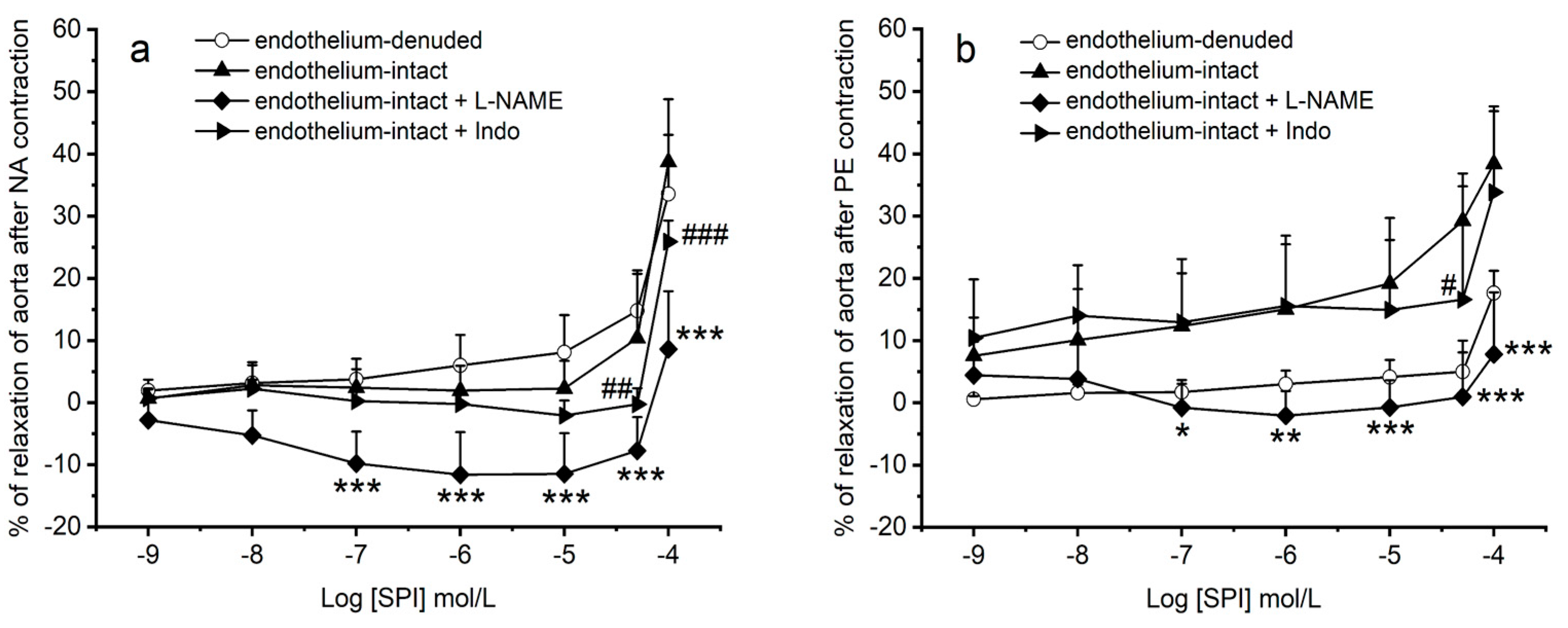
3.4. Vasodilation Induced by SPI through Endothelial Mediators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrmann, J.; Babic, M.; Tolle, M.; van der Giet, M.; Schuchardt, M. Research Models for Studying Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 2204. [Google Scholar] [CrossRef]
- Tsang, H.G.; Rashdan, N.A.; Whitelaw, C.B.A.; Corcoran, B.M.; Summers, K.M.; MacRae, V.E. Large animal models of cardiovascular disease. Cell Biochem. 2016, 34, 113–132. [Google Scholar] [CrossRef]
- Rameshrad, M.; Babaei, H.; Azarmi, Y.; Fouladi, D.F. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016, 145, 190–204. [Google Scholar] [CrossRef]
- Dejea, H.; Garcia-Canadilla, P.; Cook, A.C.; Guasch, E.; Zamora, M.; Crispi, F.; Stampanoni, M.; Bijnens, B.; Bonnin, A. Comprehensive Analysis of Animal Models of Cardiovascular Disease using Multiscale X-Ray Phase Contrast Tomography. Sci. Rep. 2019, 9, 6996. [Google Scholar] [CrossRef]
- Morales, D.R.; Morant, S.V.; MacDonald, T.M.; Mackenzie, I.S.; Doney, A.S.F.; Mitchell, L.; Bennie, M.; Robertson, C.; Hallas, J.; Pottegard, A.; et al. Impact of EMA regulatory label changes on systemic diclofenac initiation, discontinuation, and switching to other pain medicines in Scotland, England, Denmark, and The Netherlands. Pharmacoepidemiol. Drug Saf. 2020, 29, 296–305. [Google Scholar] [CrossRef]
- Ezechiáš, M.; Janochová, J.; Filipová, A.; Křesinová, Z.; Cajthaml, T. Widely used pharmaceuticals present in the environment revealed as in vitro antagonists for human estrogen and androgen receptors. Chemosphere 2016, 152, 284–291. [Google Scholar] [CrossRef]
- Sabir, S.; Akhtar, M.F.; Saleem, A. Endocrine disruption as an adverse effect of non-endocrine targeting pharmaceuticals. Environ. Sci. Pollut. Res. Int. 2019, 26, 1277–1286. [Google Scholar] [CrossRef]
- Lainscak, M.; Pelliccia, F.; Rosano, G.; Vitale, C.; Schiariti, M.; Greco, C.; Speziale, G.; Gaudio, C. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int. J. Cardiol. 2015, 200, 25–29. [Google Scholar] [CrossRef]
- Funder, J.W. Spironolactone in cardiovascular disease: An expanding universe? F1000Research 2017, 6, 1738. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Celiz, M.D.; Tso, J.; Aga, D.S. Pharmaceutical metabolites in the environment: Analytical challenges and ecological risks. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. Fetoplacental vasculature as a model to study human cardiovascular endocrine disruption. Mol. Aspects Med. 2021, 87, 101054. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Raut, S.A.; Howell, W.M.; Angus, R.A. Endocrine-disrupting effects of spironolactone in female western mosquitofish, Gambusia affinis. Environ. Toxicol. Chem. 2011, 30, 1376–1382. [Google Scholar] [CrossRef]
- Tan, H.; Chen, Q.; Hong, H.; Benfenati, E.; Gini, G.C.; Zhang, X.; Yu, H.; Shi, W. Structures of Endocrine-Disrupting Chemicals Correlate with the Activation of 12 Classic Nuclear Receptors. Environ. Sci. Technol. 2021, 55, 16552–16562. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. UV-B filter octylmethoxycinnamate-induced vascular endothelial disruption on rat aorta: In silico and in vitro approach. Chemosphere 2022, 307, 135807. [Google Scholar] [CrossRef]
- Pantan, R.; Onsa-Ard, A.; Tocharus, J.; Wonganan, O.; Suksamrarn, A.; Tocharus, C. Endothelium-independent vasorelaxation effects of 16-O-acetyldihydroisosteviol on isolated rat thoracic aorta. Life Sci. 2014, 116, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.; Lorigo, M.; Cairrao, E. Protein Interaction Network for Identifying Vascular Response of Metformin (Oral Antidiabetic). BioMedInformatics 2022, 2, 217–233. [Google Scholar] [CrossRef]
- Doggrell, S.A.; Brown, L. The spironolactone renaissance. Expert Opin. Investig. Drugs 2001, 10, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Yemisci, A.; Gorgulu, A.; Piskin, S. Effects and side-effects of spironolactone therapy in women with acne. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; Cirino, G.; Calignano, A.; Mancuso, F.; Sorrentino, L.; Andriuoli, G.; Pinto, A. Increase in the basal tone of guinea pig thoracic aorta induced by ouabain is inhibited by spironolactone canrenone and potassium canrenoate. J. Cardiovasc. Pharmacol. 1996, 28, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; Autore, G.; Cirino, G.; d’Emmanuele de Villa Bianca, R.; Calignano, A.; Vanasia, M.; Alfieri, C.; Sorrentino, L.; Pinto, A. Effect of spironolactone and its metabolites on contractile property of isolated rat aorta rings. J. Cardiovasc. Pharmacol. 2000, 36, 230–235. [Google Scholar] [CrossRef]
- Cargnelli, G.; Trevisi, L.; Debetto, P.; Luciani, S.; Bova, S. Effects of canrenone on aorta and right ventricle of the rat. J. Cardiovasc. Pharmacol. 2001, 37, 540–547. [Google Scholar] [CrossRef]
- Niazmand, S.; Fereidouni, E.; Mahmoudabady, M.; Mousavi, S.M. Endothelium-Independent Vasorelaxant Effects of Hydroalcoholic Extract from Nigella sativa Seed in Rat Aorta: The Roles of Ca2+ and K+ Channels. BioMed Res. Int. 2014, 2014, 247054. [Google Scholar] [CrossRef] [PubMed]
- Motiejunaite, J.; Amar, L.; Vidal-Petiot, E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann. D’endocrinologie 2021, 82, 193–197. [Google Scholar] [CrossRef]
- Traupe, T.; Stettler, C.D.; Li, H.; Haas, E.; Bhattacharya, I.; Minotti, R.; Barton, M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension 2007, 49, 1364–1370. [Google Scholar] [CrossRef]
- Weihua, Z.; Saji, S.; Makinen, S.; Cheng, G.; Jensen, E.V.; Warner, M.; Gustafsson, J.A. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc. Natl. Acad. Sci. USA 2000, 97, 5936–5941. [Google Scholar] [CrossRef] [PubMed]
- Nava, E.; Llorens, S. The Local Regulation of Vascular Function: From an Inside-Outside to an Outside-Inside Model. Front. Physiol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Esmaeili, H.; Sharifi, M.; Esmailidehaj, M.; Rezvani, M.E.; Hafizibarjin, Z. Vasodilatory effect of asafoetida essential oil on rat aorta rings: The role of nitric oxide, prostacyclin, and calcium channels. Phytomedicine Int. J. Phytother. Phytopharm. 2017, 36, 88–94. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, H.; Xu, H.; Wang, Y.; Hu, W.; Lou, G.; Zhang, L.; Huang, C.; Jiang, C.; Zhou, S.; et al. Inhibition of eNOS by L-NAME resulting in rat hind limb developmental defects through PFKFB3 mediated angiogenetic pathway. Sci. Rep. 2020, 10, 16754. [Google Scholar] [CrossRef]
- Suksawat, M.; Techasen, A.; Namwat, N.; Boonsong, T.; Titapun, A.; Ungarreevittaya, P.; Yongvanit, P.; Loilome, W. Inhi-bition of endothelial nitric oxide synthase in cholangiocarcinoma cell lines—A new strategy for therapy. FEBS Open Bio 2018, 8, 513–522. [Google Scholar] [CrossRef]
- Lüscher, T.F.; Yang, Z.; Tschudi, M.; von Segesser, L.; Stulz, P.; Boulanger, C.; Siebenmann, R.; Turina, M.; Bühler, F.R. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circ. Res. 1990, 66, 1088–1094. [Google Scholar] [CrossRef]
- Thorin, E.; Webb, D.J. Endothelium-derived endothelin-1. Pflug. Arch. Eur. J. Physiol. 2010, 459, 951–958. [Google Scholar] [CrossRef]
- Aldossary, H.S.; Alzahrani, A.A.; Nathanael, D.; Alhuthail, E.A.; Ray, C.J.; Batis, N.; Kumar, P.; Coney, A.M.; Holmes, A.P. G-Protein-Coupled Receptor (GPCR) Signaling in the Carotid Body: Roles in Hypoxia and Cardiovascular and Respiratory Disease. Int. J. Mol. Sci. 2020, 21, 6012. [Google Scholar] [CrossRef]
- Lucas, S. The Pharmacology of Indomethacin. Headache J. Head Face Pain 2016, 56, 436–446. [Google Scholar] [CrossRef]
- Gromotowicz-Poplawska, A.; Kloza, M.; Aleksiejczuk, M.; Marcinczyk, N.; Szemraj, J.; Kozlowska, H.; Chabielska, E. Nitric oxide as a modulator in platelet- and endothelium-dependent antithrombotic effect of eplerenone in diabetic rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2019, 70, 187–198. [Google Scholar] [CrossRef]
- Di Lullo, L.; Lavalle, C.; Scatena, A.; Mariani, M.V.; Ronco, C.; Bellasi, A. Finerenone: Questions and Answers—The Four Fundamental Arguments on the New-Born Promising Non-Steroidal Mineralocorticoid Receptor Antagonist. J. Clin. Med. 2023, 12, 3992. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Beausoleil, C.; Belcher, S.M.; Belzunces, L.P.; Emond, C.; Guerbet, M.; Rousselle, C. Non-monotonic dose-response relationships and endocrine disruptors: A qualitative method of assessment. Environ. Health 2015, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Perusquía, M.; Hernández, R.; Morales, M.A.; Campos, M.G.; Villalón, C.M. Role of endothelium in the vasodilating effect of progestins and androgens on the rat thoracic aorta. Gen. Pharmacol. 1996, 27, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Dacquet, C.; Loirand, G.; Mironneau, C.; Mironneau, J.; Pacaud, P. Spironolactone inhibition of contraction and calcium channels in rat portal vein. Br. J. Pharmacol. 1987, 92, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Jones, R.D.; Jones, T.H.; Channer, K.S.; Peers, C. Selective inhibition of L-type Ca2+ channels in A7r5 cells by physiological levels of testosterone. Endocrinology 2006, 147, 2675–2680. [Google Scholar] [CrossRef]
- Mariana, M.; Feiteiro, J.; Cairrao, E. Cardiovascular Response of Rat Aorta to Di-(2-ethylhexyl) Phthalate (DEHP) Exposure. Cardiovasc. Toxicol. 2018, 18, 356–364. [Google Scholar] [CrossRef]
- Feiteiro, J.; Mariana, M.; Verde, I.; Cairrão, E. Tributyltin Affects Rat Vascular Contractility Through L-Type Calcium Channels. Int. J. Environ. Res. 2018, 12, 215–221. [Google Scholar] [CrossRef]
- Feiteiro, J.; Mariana, M.; Gloria, S.; Cairrao, E. Inhibition of L-type calcium channels by Bisphenol A in rat aorta smooth muscle. J. Toxicol. Sci. 2018, 43, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Mizuhashi, S.; Ikegaya, Y.; Matsuki, N. Pharmacological property of tributyltin in vivo and in vitro. Environ. Toxicol. Pharmacol. 2000, 8, 205–212. [Google Scholar] [CrossRef] [PubMed]

| Vasoactive Agent | Endothelium-Denuded Rings (mN) | Endothelium-Intact Rings (mN) |
|---|---|---|
| NA 1 μmol/L | 23.39 ± 6.38 (n = 32) * | 14.68 ± 6.35 (n = 22) |
| PE 1 μmol/L | 18.47 ± 7.11 (n = 27) # | 15.40 ± 8.94 (n = 28) |
| KCl 60 mmol/L | 22.10 ± 8.27 (n = 31) | 22.55 ± 6.84 (n = 28) # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorigo, M.; Amaro, J.; Cairrao, E. Spironolactone Induces Vasodilation by Endothelium-Dependent Mechanisms Involving NO and by Endothelium-Independent Mechanisms Blocking Ca2+ Channels. J. Xenobiot. 2024, 14, 320-332. https://doi.org/10.3390/jox14010020
Lorigo M, Amaro J, Cairrao E. Spironolactone Induces Vasodilation by Endothelium-Dependent Mechanisms Involving NO and by Endothelium-Independent Mechanisms Blocking Ca2+ Channels. Journal of Xenobiotics. 2024; 14(1):320-332. https://doi.org/10.3390/jox14010020
Chicago/Turabian StyleLorigo, Margarida, João Amaro, and Elisa Cairrao. 2024. "Spironolactone Induces Vasodilation by Endothelium-Dependent Mechanisms Involving NO and by Endothelium-Independent Mechanisms Blocking Ca2+ Channels" Journal of Xenobiotics 14, no. 1: 320-332. https://doi.org/10.3390/jox14010020
APA StyleLorigo, M., Amaro, J., & Cairrao, E. (2024). Spironolactone Induces Vasodilation by Endothelium-Dependent Mechanisms Involving NO and by Endothelium-Independent Mechanisms Blocking Ca2+ Channels. Journal of Xenobiotics, 14(1), 320-332. https://doi.org/10.3390/jox14010020








