Impact of Cumulative Environmental and Dietary Xenobiotics on Human Microbiota: Risk Assessment for One Health
Abstract
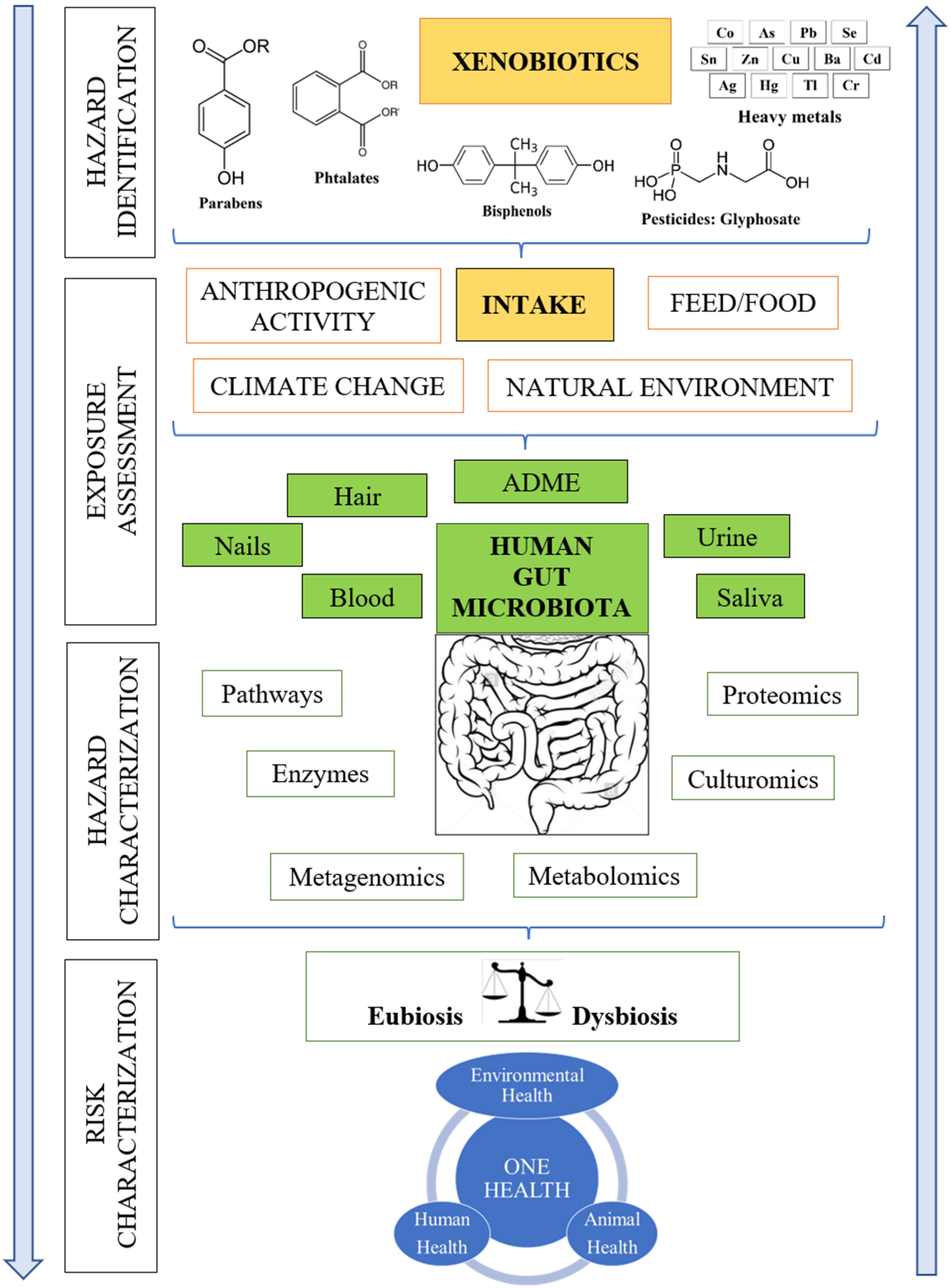
:1. Introduction
2. Key Challenges of Gut Microbial Metabolism Research
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bronzwaer, S.; Geervliet, M.; Hugas, M.; Url, B. EFSA’s Expertise Supports One Health Policy Needs. EFSA J. 2021, 19, e190501. [Google Scholar] [CrossRef] [PubMed]
- Buschhardt, T.; Günther, T.; Skjerdal, T.; Torpdahl, M.; Gethmann, J.; Filippitzi, M.-E.; Maassen, C.; Jore, S.; Ellis-Iversen, J.; Filter, M. A One Health Glossary to Support Communication and Information Exchange between the Human Health, Animal Health and Food Safety Sectors. One Health 2021, 13, 100263. [Google Scholar] [CrossRef] [PubMed]
- Ramaka, S.; Srinivas, R.; Vasudeva, M.S.; Raghuram Rao, A. Xenobiotics in Health and Disease: The Two Sides of a Coin: A Clinician’s Perspective. Open Acc. J. Toxicol. 2020, 4, 555641. [Google Scholar] [CrossRef]
- Štefanac, T.; Grgas, D.; Landeka Dragičević, T. Xenobiotics—Division and Methods of Detection: A Review. J. Xenobiot. 2021, 11, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical Transformation of Xenobiotics by the Human Gut Microbiota. Science 2017, 356, eaag2770. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.A.; Ramadan, A.T.; ElRakaiby, M.T.; Aziz, R.K. Toxicomicrobiomics: The Human Microbiome vs. Pharmaceutical, Dietary, and Environmental Xenobiotics. Front. Pharmacol. 2020, 11, 390. [Google Scholar] [CrossRef]
- Aziz, R.K. Toxicomicrobiomics: Narrowing the Gap between Environmental and Medicinal Toxicogenomics. OMICS A J. Integr. Biol. 2018, 22, 788–789. [Google Scholar] [CrossRef]
- Aguilera, M.; Gálvez-Ontiveros, Y.; Rivas, A. Endobolome, a New Concept for Determining the Influence of Microbiota Disrupting Chemicals (MDC) in Relation to Specific Endocrine Pathogenesis. Front. Microbiol. 2020, 11, 578007. [Google Scholar] [CrossRef]
- Larsson, K.; Lindh, C.H.; Jönsson, B.A.; Giovanoulis, G.; Bibi, M.; Bottai, M.; Bergström, A.; Berglund, M. Phthalates, Non-Phthalate Plasticizers and Bisphenols in Swedish Preschool Dust in Relation to Children’s Exposure. Environ. Int. 2017, 102, 114–124. [Google Scholar] [CrossRef]
- Lei, M.; Menon, R.; Manteiga, S.; Alden, N.; Hunt, C.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Environmental Chemical Diethylhexyl Phthalate Alters Intestinal Microbiota Community Structure and Metabolite Profile in Mice. mSystems 2019, 4, e00724-19. [Google Scholar] [CrossRef] [Green Version]
- Adamovsky, O.; Buerger, A.N.; Vespalcova, H.; Sohag, S.R.; Hanlon, A.T.; Ginn, P.E.; Craft, S.L.; Smatana, S.; Budinska, E.; Persico, M.; et al. Evaluation of Microbiome-Host Relationships in the Zebrafish Gastrointestinal System Reveals Adaptive Immunity Is a Target of Bis(2-Ethylhexyl) Phthalate (DEHP) Exposure. Environ. Sci. Technol. 2020, 54, 5719–5728. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, Q.; Tian, P.; Wang, L.; Li, X.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Gut Microbiota Dysbiosis Might Be Responsible to Different Toxicity Caused by Di-(2-Ethylhexyl) Phthalate Exposure in Murine Rodents. Environ. Pollut. 2020, 261, 114164. [Google Scholar] [CrossRef] [PubMed]
- Sabra, S.; Malmqvist, E.; Saborit, A.; Gratacós, E.; Gomez Roig, M.D. Heavy Metals Exposure Levels and Their Correlation with Different Clinical Forms of Fetal Growth Restriction. PLoS ONE 2017, 12, e0185645. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xu, P.; Huang, C.; Liu, G.; Chen, S.; Hu, G.; Li, G.; Liu, P.; Guo, X. Effects of Subchronic Exposure of Mercuric Chloride on Intestinal Histology and Microbiota in the Cecum of Chicken. Ecotoxicol. Environ. Saf. 2020, 188, 109920. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, C.; Wu, C.; Guo, X.; Hu, G.; Wu, Q.; Xu, Z.; Li, G.; Cao, H.; Li, L.; et al. Subchronic Oral Mercury Caused Intestinal Injury and Changed Gut Microbiota in Mice. Sci. Total Environ. 2020, 721, 137639. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hartmann, E.M.; Kline, J.; Van Den Wymelenberg, K.; Halden, R.U. Assessment of Human Exposure to Triclocarban, Triclosan and Five Parabens in U.S. Indoor Dust Using Dispersive Solid Phase Extraction Followed by Liquid Chromatography Tandem Mass Spectrometry. J. Hazard Mater. 2018, 360, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Alcalá, L.; Buckley, J.P.; Boyle, M. Parabens and Measures of Adiposity among Adults and Children from the U.S. General Population: NHANES 2007–2014. Int. J. Hyg. Environ. Health 2018, 221, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Poston, R.G.; Saha, R.N. Epigenetic Effects of Polybrominated Diphenyl Ethers on Human Health. Int. J. Environ. Res. Public Health 2019, 16, 2703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hu, C.; Lai, N.L.-S.; Zhang, W.; Hua, J.; Lam, P.K.S.; Lam, C.W.J.; Zhou, B. Acute Exposure to PBDEs at an Environmentally Realistic Concentration Causes Abrupt Changes in the Gut Microbiota and Host Health of Zebrafish. Environ. Pollut. 2018, 240, 17–26. [Google Scholar] [CrossRef]
- Yuan, X.; Pan, Z.; Jin, C.; Ni, Y.; Fu, Z.; Jin, Y. Gut Microbiota: An Underestimated and Unintended Recipient for Pesticide-Induced Toxicity. Chemosphere 2019, 227, 425–434. [Google Scholar] [CrossRef]
- Gillezeau, C.; Alpert, N.; Joshi, P.; Taioli, E. Urinary Dialkylphosphate Metabolite Levels in US Adults—National Health and Nutrition Examination Survey 1999–2008. Int. J. Environ. Res. Public Health 2019, 16, 4605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.-W.; Fang, B.; Pang, G.-F.; Zhang, M.; Ren, F.-Z. Age- and Diet-Specific Effects of Chronic Exposure to Chlorpyrifos on Hormones, Inflammation and Gut Microbiota in Rats. Pestic. Biochem. Physiol. 2019, 159, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Mendler, A.; Geier, F.; Haange, S.-B.; Pierzchalski, A.; Krause, J.L.; Nijenhuis, I.; Froment, J.; Jehmlich, N.; Berger, U.; Ackermann, G.; et al. Mucosal-Associated Invariant T-Cell (MAIT) Activation Is Altered by Chlorpyrifos- and Glyphosate-Treated Commensal Gut Bacteria. J. Immunotoxicol. 2020, 17, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manabe, M.; Kanda, S.; Fukunaga, K.; Tsubura, A.; Nishiyama, T. Evaluation of the Estrogenic Activities of Some Pesticides and Their Combinations Using MtT/Se Cell Proliferation Assay. Int. J. Hyg. Environ. Health 2006, 209, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Bian, X.; Mahbub, R.; Lu, K. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ. Health Perspect. 2017, 125, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhan, J.; Liu, D.; Luo, M.; Han, J.; Liu, X.; Liu, C.; Cheng, Z.; Zhou, Z.; Wang, P. Organophosphorus Pesticide Chlorpyrifos Intake Promotes Obesity and Insulin Resistance through Impacting Gut and Gut Microbiota. Microbiome 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Chen, C.; Feng, Y.; Ren, N.; Sun, K. Effects of Cadmium on Intestinal Histology and Microbiota in Freshwater Crayfish (Procambarus clarkii). Chemosphere 2020, 242, 125105. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Government of the United Kingdom. Available online: https://apo.org.au/node/63983 (accessed on 2 March 2022).
- Lindell, A.E.; Zimmermann-Kogadeeva, M.; Patil, K.R. Multimodal Interactions of Drugs, Natural Compounds and Pollutants with the Gut Microbiota. Nat. Rev. Microbiol. 2022, 6, 235. [Google Scholar] [CrossRef]
- Penders, J.; Stobberingh, E.; Savelkoul, P.; Wolffs, P. The Human Microbiome as a Reservoir of Antimicrobial Resistance. Front. Microbiol. 2013, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Anthony, W.E.; Burnham, C.A.D.; Dantas, G.; Kwon, J.H. The Gut Microbiome as a Reservoir for Antimicrobial Resistance. J. Infect. Dis. 2021, 223, S209–S213. [Google Scholar] [CrossRef]
- Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control). OJ L 334, 17.12.2010, pp. 17–119. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0075 (accessed on 14 December 2021).
- Apel, P.; Rousselle, C.; Lange, R.; Sissoko, F.; Kolossa-Gehring, M.; Ougier, E. Human Biomonitoring Initiative (HBM4EU)—Strategy to Derive Human Biomonitoring Guidance Values (HBM-GVs) for Health Risk Assessment. Int. J. Hyg. Environ. Health 2020, 230, 113622. [Google Scholar] [CrossRef] [PubMed]
- Eshel, G.; Shepon, A.; Makov, T.; Milo, R. Land, Irrigation Water, Greenhouse Gas, and Reactive Nitrogen Burdens of Meat, Eggs, and Dairy Production in the United States. Proc. Natl. Acad. Sci. USA 2014, 111, 11996–12001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Raikhel, V.; Gopalakrishnan, K.; Fernandez-Hernandez, H.; Lambertini, L.; Manservisi, F.; Falcioni, L.; Bua, L.; Belpoggi, F.; Teitelbaum, S.L.; et al. Effect of Postnatal Low-Dose Exposure to Environmental Chemicals on the Gut Microbiome in a Rodent Model. Microbiome 2016, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Tripathi, P. Gut Microbiome and Type 2 Diabetes: Where We Are and Where to Go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsi, V.; Didagelos, M.; Skevofilax, S.; Armenis, I.; Kartalis, A.; Vlachopoulos, C.; Karvounis, H.; Tousoulis, D. GUT Microbiome-GUT Dysbiosis-Arterial Hypertension: New Horizons. Curr. Hypertens. Rev. 2019, 15, 40–46. [Google Scholar] [CrossRef]
- Liess, M.; Henz, S.; Shahid, N. Modeling the Synergistic Effects of Toxicant Mixtures. Environ. Sci. Eur. 2020, 32, 119. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Wang, G.; Han, R.; Xie, X. Effects of Chlorpyrifos on the Gut Microbiome and Urine Metabolome in Mouse (Mus musculus). Chemosphere 2016, 153, 287–293. [Google Scholar] [CrossRef]
- Silbergeld, E.K. The Microbiome: Modulator of Pharmacological and Toxicological Exposures and Responses. Toxicol. Pathol. 2017, 45, 190–194. [Google Scholar] [CrossRef]
- Malaisé, Y.; Ménard, S.; Cartier, C.; Lencina, C.; Sommer, C.; Gaultier, E.; Houdeau, E.; Guzylack-Piriou, L. Consequences of Bisphenol a Perinatal Exposure on Immune Responses and Gut Barrier Function in Mice. Arch. Toxicol. 2017, 7, 14472. [Google Scholar] [CrossRef] [PubMed]
- Graw, S.; Chappell, K.; Washam, C.L.; Gies, A.; Bird, J.; Robeson, M.S.; Byrum, S.D. Multi-Omics Data Integration Considerations and Study Design for Biological Systems and Disease. Mol. Omics 2021, 17, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.-I.; McDonald, D.; et al. Best Practices for Analysing Microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzorati, M.; Van de Wiele, T. An Advanced In Vitro Technology Platform to Study the Mechanism of Action of Prebiotics and Probiotics in the Gastrointestinal Tract. J. Clin. Gastroenterol. 2016, 50, S124. [Google Scholar] [CrossRef]
- Dietert, R.R. The Microbiome in Early Life: Self-Completion and Microbiota Protection as Health Priorities. Birth Defects Res. B Dev. Reprod. Toxicol. 2014, 101, 333–340. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, A.; Acuña, I.; Torres-Sánchez, A.; Ruiz-Moreno, Á.; Cerk, K.; Rivas, A.; Suárez, A.; Monteoliva-Sánchez, M.; Aguilera, M. Next Generation Probiotics for Neutralizing Obesogenic Effects: Taxa Culturing Searching Strategies. Nutrients 2021, 13, 1617. [Google Scholar] [CrossRef] [PubMed]
- Ben, G.H.; Bouri, M.; Mougou Hamdane, A.; Schuster, C.; Leclerque, A.; Rhouma, A. Bacillus Velezensis Strain MBY2, a Potential Agent for the Management of Crown Gall Disease. PLoS ONE 2021, 16, e0252823. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- De Gregorio, V.; Telesco, M.; Corrado, B.; Rosiello, V.; Urciuolo, F.; Netti, P.A.; Imparato, G. Intestine-Liver Axis On-Chip Reveals the Intestinal Protective Role on Hepatic Damage by Emulating Ethanol First-Pass Metabolism. Front. Bioeng. Biotechnol. 2020, 8, 163. [Google Scholar] [CrossRef]
- López-Moreno, A.; Aguilera, M. Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Nutrients 2020, 12, 757. [Google Scholar] [CrossRef] [Green Version]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal Microbiota of 6-Week-Old Infants across Europe: Geographic Influence beyond Delivery Mode, Breast-Feeding, and Antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Humboldt-Dachroeden, S.; Rubin, O.; Sylvester Frid-Nielsen, S. The State of One Health Research across Disciplines and Sectors—A Bibliometric Analysis. One Health 2020, 10, 100146. [Google Scholar] [CrossRef] [PubMed]
- Essack, S.Y. Environment: The Neglected Component of the One Health Triad. Lancet Planet Health 2018, 2, 238–239. [Google Scholar] [CrossRef]
- Farm to Fork Strategy. European Commission. Available online: https://ec.europa.eu/food/horizontal-topics/farm-fork-strategy_es (accessed on 2 March 2022).
- Caring for Soil Is Caring for Life. Available online: https://ec.europa.eu/info/publications/caring-soil-caring-life_en (accessed on 2 March 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, P.; Torres-Sánchez, A.; López-Moreno, A.; Cerk, K.; Ruiz-Moreno, Á.; Monteoliva-Sánchez, M.; Ampatzoglou, A.; Aguilera, M.; Gruszecka-Kosowska, A. Impact of Cumulative Environmental and Dietary Xenobiotics on Human Microbiota: Risk Assessment for One Health. J. Xenobiot. 2022, 12, 56-63. https://doi.org/10.3390/jox12010006
Ortiz P, Torres-Sánchez A, López-Moreno A, Cerk K, Ruiz-Moreno Á, Monteoliva-Sánchez M, Ampatzoglou A, Aguilera M, Gruszecka-Kosowska A. Impact of Cumulative Environmental and Dietary Xenobiotics on Human Microbiota: Risk Assessment for One Health. Journal of Xenobiotics. 2022; 12(1):56-63. https://doi.org/10.3390/jox12010006
Chicago/Turabian StyleOrtiz, Pilar, Alfonso Torres-Sánchez, Ana López-Moreno, Klara Cerk, Ángel Ruiz-Moreno, Mercedes Monteoliva-Sánchez, Antonis Ampatzoglou, Margarita Aguilera, and Agnieszka Gruszecka-Kosowska. 2022. "Impact of Cumulative Environmental and Dietary Xenobiotics on Human Microbiota: Risk Assessment for One Health" Journal of Xenobiotics 12, no. 1: 56-63. https://doi.org/10.3390/jox12010006
APA StyleOrtiz, P., Torres-Sánchez, A., López-Moreno, A., Cerk, K., Ruiz-Moreno, Á., Monteoliva-Sánchez, M., Ampatzoglou, A., Aguilera, M., & Gruszecka-Kosowska, A. (2022). Impact of Cumulative Environmental and Dietary Xenobiotics on Human Microbiota: Risk Assessment for One Health. Journal of Xenobiotics, 12(1), 56-63. https://doi.org/10.3390/jox12010006









