Psilocybin for Treating Psychiatric Disorders: A Psychonaut Legend or a Promising Therapeutic Perspective?
Abstract
:1. Introduction
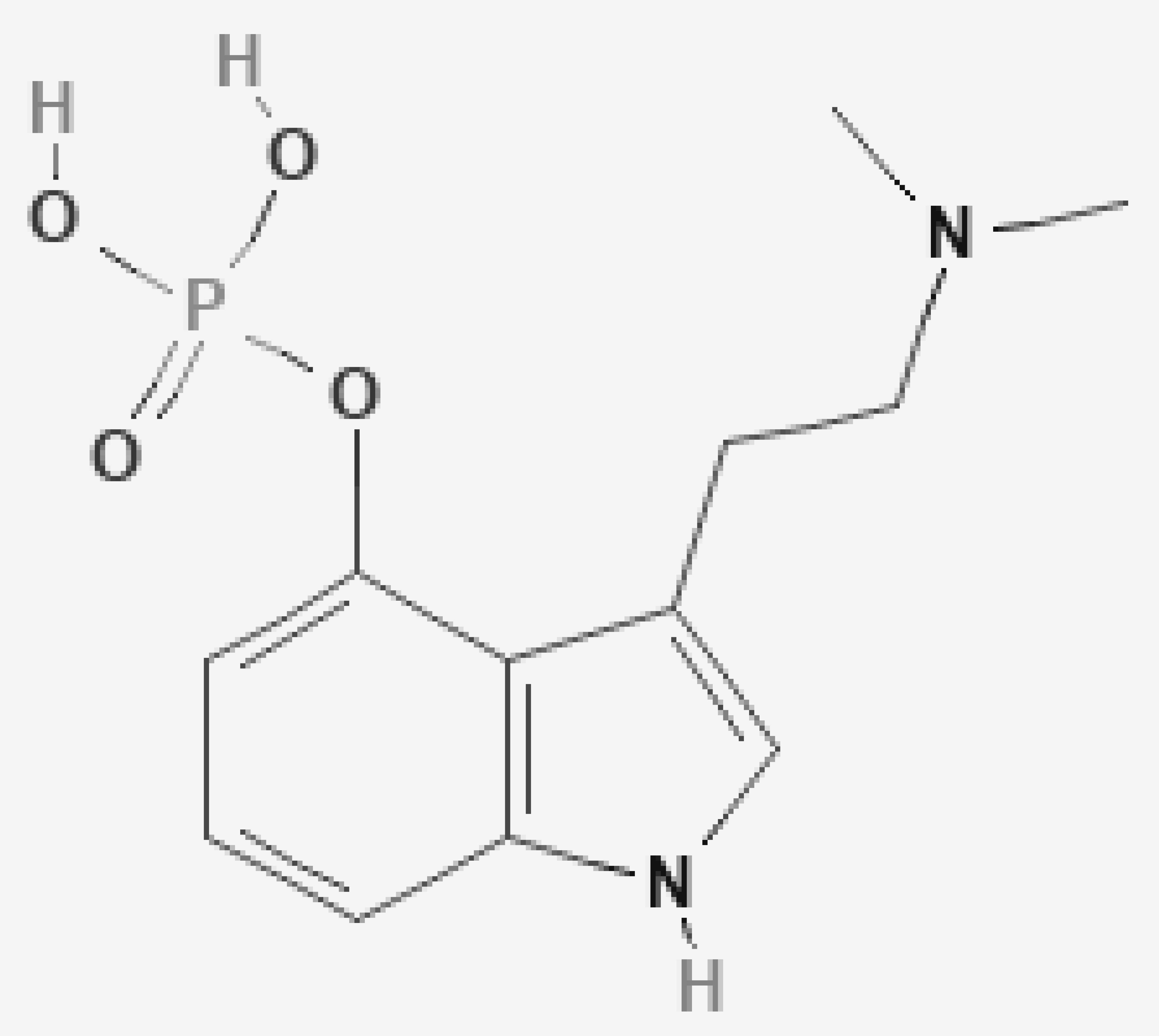
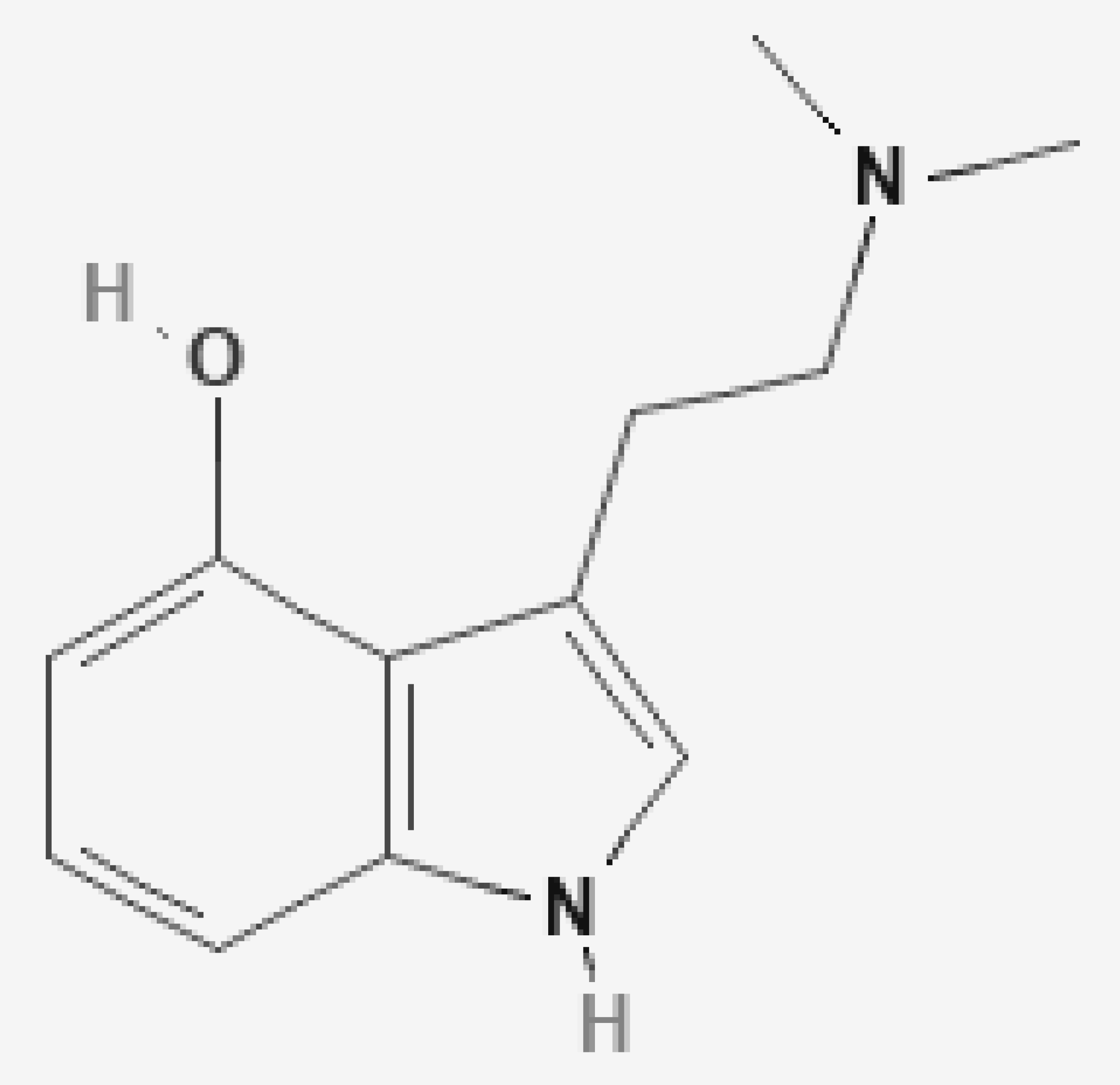
2. Chemistry
3. Pharmacokinetics
4. Pharmacodynamic
5. Functional Studies
6. Toxicity
7. Psilocybin and Mood Disorders
8. Psilocybin and Obsessive–Compulsive Disorder
9. Psilocybin and Addiction
10. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nutt, D. Psychedelic drugs—A new era in psychiatry? Dialogues Clin. Neurosci. 2019, 21, 139–147. [Google Scholar]
- Bruhn, J.G.; de Smet, P.A.; El-Seedi, H.R.; Beck, O. Mescaline use for 5700 years. Lancet 2002, 359, 1866. [Google Scholar] [CrossRef]
- Akers, B.P.; Ruiz, J.F.; Piper, A.; Ruck, C.A.P. A prehistoric mural in Spain depicting neurotropic psilocybe mushrooms? Econ. Bot. 2011, 65, 121–128. [Google Scholar] [CrossRef]
- Prentiss, D.W.; Morgan, F.P. Anhalonium lewinii (mescal buttons). Ther. Gaz. 1895, 11, 577–585. [Google Scholar]
- Jay, M. Mescaline: A Global History of the First Psychedelic; Yale University Press: New Haven, CT, USA, 2019; pp. 1–304. [Google Scholar]
- Hofmann, A. LSD—My Problem Child; Oxford University Press: Oxford, UK, 2019; pp. 1–256. [Google Scholar]
- Grinspoon, L.; Bakalar, J. The psychedelic drug terapie. Curr. Psychiatr. Ther. 1981, 20, 275–283. [Google Scholar] [PubMed]
- Sercl, M.; Kovarik, J.; Jaros, O. Clinical experiences with psilocybin Sandoz. Sb. Ved. Pr. Lek. Fak. Karlov. Univ. V Hradci Kral. 1961, 4, 421–426. [Google Scholar]
- Heimann, H. On the treatment of therapy-resistant neuroses with model psychoses (psilocybin). Schweiz Arch. Neurol. Neurochir. Psychiatr. 1962, 89, 214–220. [Google Scholar]
- Reda, G.C.; Vella, G.; Cancrini, L.; D’Agostino, E. Clinical and psychopathological study of psilocybin. Riv. Sper. Freniatr. Med. Leg. Alien. Ment. 1964, 88, 7–76. [Google Scholar] [PubMed]
- Delay, J.; Pichot, P.; Lemperiere, T.; Quetin, A.M. Therapeutic effect of psilocybin on convulsive neurosis. Ann. Med. Psychol. 1959, 117, 509–515. [Google Scholar]
- Duche, D.J. The effects of psilocybin in a case of hysteria. Sem. Hop. 1961, 37, 3061–3062. [Google Scholar]
- Růzicková, R.; Bílý, D.; Vyhnánková, M.; Dubanský, B.; Konias, V.; Soucek, Z. Effect of psilocybine in chronic schizophrenias. I. Clinical findings. Ceskoslov. Psychiatr. 1967, 63, 158–165. [Google Scholar]
- Fisher, G. The psycholytic treatment of a childhood schizophrenic girl. Int. J. Soc. Psychiatry 1970, 16, 112–130. [Google Scholar] [CrossRef]
- Mentzner, R. Sacred Mushroom of Visions: Teonanacatl: A Sourcebook on the Psilocybin Mushroom; Mentzer, R., Ed.; Simon and Schuster Park Street Press: Rochester, VT, USA, 2005; pp. 1–293. [Google Scholar]
- Keeler, M.H.; Reifler, C.B. Suicide during an LSD reaction. Am. J. Psychiatr. 1967, 123, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Rucker, J.J.H.; Iliff, J.; Nutt, D.J. Psychiatry & psychedelic drugs. Past, present & future. Neuropharmacology 2018, 142, 200–218. [Google Scholar] [PubMed]
- Tyls, F.; Palenicek, T.; Horacek, J. Psilocybin- Summary of knowledge and new perspectives. Eur. Neuropsychopharmacol. 2014, 24, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Cody, J.T. Chapter 4 Hallucinogenes. In Handbook of Analytical Separations; Bogusz, M.J., Science, B.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 175–201. [Google Scholar]
- StiJve, T. Worldwide occurrence of psychoactive mushrooms-an update. Czen Mycol. 1995, 48, 11–19. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. Alkaloids in the nature: Pharmacological applications in clinical practice of berberine and mate tea. Curr. Top. Med. Chem. 2014, 14, 200–206. [Google Scholar] [CrossRef]
- Tittarelli, R.; Mannocchi, G.; Pantano, F.; Romolo, F.S. Recreational use, analysis and toxicity of tryptomines. Curr. Neuropharmacol. 2015, 13, 26–46. [Google Scholar] [CrossRef] [Green Version]
- Cayman Chemicals. Psilocybin. Available online: https://www.caymanchem.com/product/14041/psilocybin (accessed on 9 November 2020).
- Ballesteros, S.; Ramon, M.F.; Iturralde, M.J.; Martinez-Arrieta, R. Natural source of drugs of abuse: Magic mushrooms. In New Research on Street Drugs; Cole, S.M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2006; pp. 167–186. [Google Scholar]
- Pendersen-Bjergaard, S.; Sannes, E.; Rasmussen, K.E.; Tonnesen, F. Determination of psilocybin in Psilocybe semilanceata by capillary zone electrophoresis. J. Chromatogr. Biomed. Sci. Appl. 1997, 694, 375–381. [Google Scholar] [CrossRef]
- van Amsterdam, J.; Opperhuizen, A.; van den Brink, W. Harm potential of magic mushroom use: A review. Regul Toxicol. Pharmacol. 2011, 59, 423–429. [Google Scholar] [CrossRef]
- Hopf, A.; Eckert, H. Distribution patterns of 14C-psilocin in the brains of various animals. Act. Nerv. Supp. 1974, 16, 64–66. [Google Scholar]
- Law, F.C.P.; Poon, G.; Chui, Y.C.; He, S.H. 14C-Psilocin tissue distribution in pregnant rats after intravenous administration. Funct. Foods Health Dis. 2014, 4, 232–244. [Google Scholar] [CrossRef] [Green Version]
- Hasler, F.; Bourquin, D.; Brenneisen, R.; Bär, T.; Vollenweider, F.X. Determination of psilocin and 4-hydroxyindole-3-yl-acetic-acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm. Acta Helv. 1997, 72, 175–184. [Google Scholar] [CrossRef]
- Hasler, F.; Bourquin, D.; Brenneisen, R.; Vollenweider, F.X. Renal excretion profiles of psilocin following oral administration of psilocybin: A controller study in man. J. Pharm. Biomed. Anal. 2002, 30, 331–339. [Google Scholar] [CrossRef]
- Eivindvik, K.; Rasmussen, K.E.; Sund, R.B. Handling of psilocybin and psilocin by everted sacs of rat jejunum and colon. Acta Pharm. Nord. 1989, 1, 295–302. [Google Scholar] [PubMed]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Helsley, S.; Fiorella, D.; Rabin, R.A.; Winter, J.C. A comparison of N,N-dimethyltryptamine, harmaline, and selected congeners in rats trained with LSD as a discriminative stimulus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1998, 22, 649–663. [Google Scholar] [CrossRef]
- Kalberer, F.; Kreis, W.; Rutschmann, J. The fate of psilocin in the rat. Biochem. Pharmachol. 1962, 11, 261–269. [Google Scholar] [CrossRef]
- Lindenblatt, H.; Kramer, E.; Holzmann-Erens, P.; Gouzoulis-Mayfrank, E.; Kovar, K.A. Quantitation of psilocin in human plasma by high-performance liquid chromatography and electrochemical detection: Comparison of liquid-liquid extraction with automated on-line solid-phase extraction. J. Chromatogr. B Biomed. Sci. Appl. 1998, 709, 255–263. [Google Scholar] [CrossRef]
- Martin, R.; Schurenkamp, J.; Gasse, A. Determination of psilocin, bufotenine, LSD, and its metabolites in serum, plasma and urine by SPE-LC-MS/MS. Int. J. Legal Med. 2013, 127, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, A.F.; Moore, K.A.; Levine, B. The detection of psilocin in human urine. J. Forensic Sci. 2001, 46, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Sticht, G.; Kaferstein, H. Detection of psilocin in body fluids. Forensic Sci. Int. 2000, 113, 403–407. [Google Scholar] [CrossRef]
- Manevski, N.; Kurkela, M.; Hoglund, C.; Mauriala, T.; Court, M.H.; Yli-Kauhaluoma, J.; Finel, M. Glucuronidation of psilocin and 4-hydroxyindole by the human UDP-glucuronosyltransferases. Drug Metab. Dispos. 2010, 38, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Kovacic, P. Unifying electron transfer mechanism for psilocybin and psilocin. Med. Hypotheses 2009, 73, 626. [Google Scholar] [CrossRef]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef]
- Ray, T.S. Psychedelics and the human receptorome. PLoS ONE 2010, 5, e9019. [Google Scholar] [CrossRef]
- Creese, I.; Burt, D.R.; Snyder, S.H. The dopamine receptor: Different binding of d-LSD and related agents to agonist and antagonist states. Life Sci. 1975, 17, 15–20. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vollenweider-Scherpenhuysen, M.F.I.; Babler, A.; Vogel, H.; Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via serotonin-2 agonist action. Neuroreport 1998, 9, 3897–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollenweider, F.X.; Vontobel, P.; Hell, D.; Leenders, K.L. 5-HAT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man-a PET study with [11C] raclopride. Neuropsychopharmacology 1999, 112, 424–434. [Google Scholar] [CrossRef] [Green Version]
- McKenn, D.J.; Repke, D.B.; Lo, L.; Peroutka, S.J. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 1990, 29, 193–198. [Google Scholar] [CrossRef]
- Horita, A. Some biochemical studies on psilocybin and psilocin. J. Neuropsychiatr. 1963, 4, 370–373. [Google Scholar]
- Fink, M. EEG and human psychopharmacology. Annu. Rev. Pharmacol. 1969, 9, 241–258. [Google Scholar] [CrossRef]
- Horibe, M. The effects of psilocybin on EEG and behaviour in monkeys. Act. Nerv. Super. 1974, 16, 40–42. [Google Scholar]
- Meldrum, B.S.; Naquet, R. Effects of psilocybin, dimethyl-tryptamine, mescaline and various lysergic acid derivatives on the EEG and on photically induced epilepsy in the baboon (Papio papio). Electroencephalogr. Clin. Neurophysiol. 1971, 31, 563–572. [Google Scholar] [CrossRef]
- Da Fonseca, J.S.; Cardoso, C.; Salgueiro, E.; Fialho, M.L. Neuro-physiological and psychological study of psilocybin-induced modification of visual information processing in man. Neuro Psychopharmacol. 1965, 4, 315–319. [Google Scholar]
- Rynearson, R.R.; Wilson, M.R., Jr.; Bickford, R.G. Psilocybin-induced changes in psychologic function, electroencephalogram, and light-evoked potentials in humans ubjects. Mayo Clin. Proc. 1968, 43, 191–204. [Google Scholar] [PubMed]
- Kometer, M.; Schmidt, A.; Jäncke, L.; Vollenweider, F.X. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J. Neurosci. 2013, 33, 10544–10551. [Google Scholar] [CrossRef] [Green Version]
- Muthukumaraswamy, S.D.; Carhart-Harris, R.L.; Moran, R.J.; Brookes, M.J.; Williams, T.M.; Errtizoe, D.; Sessa, B.; Papadopoulos, A.; Bolstridge, M.; Singh, K.D.; et al. Broadband Cortical Desynchronization Underlies the Human Psychedelic State. J. Neurosci. 2013, 33, 15171–15183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollenweidera, F.X.; Leendersb, K.L.; Scharfettera, C.; Maguire, P.; Stadelmann, O.; Angsta, J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 1997, 16, 357–372. [Google Scholar] [CrossRef]
- Gouzoulis-Mayfrank, E.; Schreckenberger, M.; Sabri, O.; Arning, C.; Thelen, B.; Spitzer, M.; Kovar, K.A.; Hermle, L.; Büll, U.; Sass, H. Neurometabolic effects of psilocybin, 3, 4 methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology 1999, 20, 565–581. [Google Scholar] [CrossRef] [Green Version]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.R.; Preller, K.H.; Kraehenmann, R.; Michels, L.; Staempfli, P.; Vollenweider, F.X. Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. NeuroImage 2017, 159, 70–78. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Williams, T.M.; Erritzoe, D.; Abbasi, N.; Bargiotas, T.; Hobden, P.; Sharp, D.J.; Evans, J.; Feilding, A.; et al. Implications for psychedelic-assisted psychotherapy: Functional magnetic resonance imaging study with psilocybin. Br. J. Psychiatry 2012, 200, 238–244. [Google Scholar] [CrossRef]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 2019, 44, 1328–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenbæk, D.S.; Madsen, M.K.; Ozenne, B.; Kristiansen, S.; Burmester, D.; Erritzoe, D.; Knudsen, G.M.; Fisher, P.M. Brain serotonin 2A receptor binding predicts subjective temporal and mystical effects of psilocybin in healthy humans. J. Psychopharmacol. 2021, 35, 459–468. [Google Scholar] [CrossRef]
- Madsen, M.K.; Fishera, P.M.; Stenbæka, D.S.; Kristiansen, S.; Burmester, D.; Lehelc, S.; Páleníček, T.; Kuchařde, M.; Svarera, C.; Ozenne, B.; et al. A single psilocybin dose is associated with long-term increased mindfulness, preceded by a proportional change in neocortical 5-HT2A receptor binding. Eur. Neuropsychopharmacol. 2020, 33, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Knoblauch, J.; FrazeeII, C.; Baron, A.; Dudley, M. Accidental death involving psilocin from ingesting “magic mushroom”. In Toxicology Cases for the Clinical and Forensic Laboratory; Ketha, H., Garg, U., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 379–382. [Google Scholar]
- Gerault, A.; Picart, D. Intoxication mortelle a la suite de la consommation volontaire et en groupe de champignons hallucinogenes. Bull. Soc. Mycol. Fr. 1996, 112, 1–14. [Google Scholar]
- PubChem. Psilocyn. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10624#section=Acute-Effects&fullscreen=true (accessed on 28 January 2021).
- PubChem. Psilocin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4980#section=Hazard-Classes-and-Categories (accessed on 28 January 2021).
- Harris, I.; Wolbach, A.B.; Wikler, A.; Miner, E.J. Cross tolerance between LSD and psilocybin. Psychopharmacologia 1961, 2, 147–149. [Google Scholar]
- Geyer, M.A.; Vollenweider, F.X. Serotonin research: Contributions to under standing psychoses. Trends Pharmacol. Sci. 2008, 29, 445–453. [Google Scholar] [CrossRef]
- Hasler, F.; Grimberg, U.; Benz, M.A.; Huber, T.; Vollenweider, F.X. Acute psychological and physiological effects of psilocybin in healthy humans: A double-blind placebo-controlled dose effect study. Psychopharmacology 2004, 172, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Hollister, L.E. Clinical, biochemical and psychologic effects of psilocybin. Arch. Int. Pharmacodyn. Ther. 1961, 130, 42–52. [Google Scholar] [PubMed]
- Halpern, J.H. Hallucinogenes and dissociative agents naturally growing in the United States. Pharmacol. Ther. 2004, 102, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Denis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef]
- Fowler, J.S.; Volkow, N.D.; Wang, G.J.; Pappas, N.; Logan, J.; MacGregor, R.; Alexoff, D.; Shea, C.; Schlyer, D.; Wolf, A.P.; et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature 1996, 379, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.; Myronc, H.S.; Summerbell, R.K.; Mackenzie, C.A. Acute renal injury cause by confirmed Psilocybe cubensis mushroom ingestion. Med. Mycol. Case Rep. 2019, 23, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Peden, N.; Bisset, A.F.; Macaulay, K.E.C.; Crooks, J. Clinical toxicology of ‘magic mushroom’ ingestion. Postgrad. Med. J. 1981, 57, 543–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.; Richards, W.; Griffiths, R. Human hallucinogen research: Guideline for safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.R.; Preller, K.H.; Braden, B.B.; Riecken, C.; Vollenweider, F.X. Rostral anterior cingulate thickness predicts the emotional psilocybin experience. Biomedicines 2020, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Strassman, R.J. Adverse reactions to psychedelic drugs. A review of the literature. J. Ment. Dis. 1984, 172, 577–595. [Google Scholar] [CrossRef] [PubMed]
- van Went, G.F. Mutagenicity testing of 3 hallucinogenes: LSD, psilocybin and delta-9-THC, using the micronucleus test. Experientia 1978, 34, 324–325. [Google Scholar] [CrossRef]
- Grob, C.S.; Danforth, A.L.; Chopra, G.S.; Hagerty, M.; McKay, C.R.; Halberstadt, A.L.; Greer, G.R. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch. Gen. Psychiatry 2011, 68, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef] [Green Version]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of psilocybin-Assisted therapy on major depressive disorder. A randomized clinical trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D. Trial of psilocybin versus escitalopram for depression. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J. Clin. Psychiatry 2006, 67, 1735–1740. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, J.A. Psilocybin and obsessive compulsive disorder. J. Psychoact. Drugs 2014, 46, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Leonard, H.L.; Rapoport, J.L. Relief of obsessive-compulsive symptoms by LSD and psilocin. Am. J. Psychiatry 1987, 144, 1239–1240. [Google Scholar]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.C.R.; Strassman, R.J. Psilocybin assisted treatment for alcohol dependance: A proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Garcia-Romeu, A.; Cosimano, M.P.; Griffiths, R.R. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 2014, 28, 983–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Romeu, A.; Griffiths, R.R.; Johnson, M.W. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr. Drug Abuse Rev. 2014, 7, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIH. U.S. National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04161066 (accessed on 23 February 2021).
- Muthukumaraswamy, S.; Forsyth, A.; Lumley, T. Blinding and expectancy confounds in psychedelic randomised controlled trials. Expert Rev. Clin. Pharmacol. 2021, 14, 1133–1152. [Google Scholar] [CrossRef]
| Central Nervous System | Dream-like state, illusions, hallucinations, synesthesiae, paraesthesia altered state of consciousness, altered self-perception, derealization, depersonalization, altered perception of time and space, altered mood, altered concentration, delusions or unusual ideas, altered emoziona state, euphoria, panic attacks, convulsions, headache, verigo, flushing. |
| Visual System | Mydriasis |
| Cardiovascular System | Achicardia, hypertension, hypotension |
| Respiratory System | Hypoxemia |
| Gastrointestinal System | Nauseas, vomiting, abdominal pain |
| Renal System | Urinary incontinence, renal failure |
| Musculoskeletal System | Muscle weakness |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, M.; Bevione, F.; Mondola, R. Psilocybin for Treating Psychiatric Disorders: A Psychonaut Legend or a Promising Therapeutic Perspective? J. Xenobiot. 2022, 12, 41-52. https://doi.org/10.3390/jox12010004
Coppola M, Bevione F, Mondola R. Psilocybin for Treating Psychiatric Disorders: A Psychonaut Legend or a Promising Therapeutic Perspective? Journal of Xenobiotics. 2022; 12(1):41-52. https://doi.org/10.3390/jox12010004
Chicago/Turabian StyleCoppola, Maurizio, Francesco Bevione, and Raffaella Mondola. 2022. "Psilocybin for Treating Psychiatric Disorders: A Psychonaut Legend or a Promising Therapeutic Perspective?" Journal of Xenobiotics 12, no. 1: 41-52. https://doi.org/10.3390/jox12010004
APA StyleCoppola, M., Bevione, F., & Mondola, R. (2022). Psilocybin for Treating Psychiatric Disorders: A Psychonaut Legend or a Promising Therapeutic Perspective? Journal of Xenobiotics, 12(1), 41-52. https://doi.org/10.3390/jox12010004






