Practical Challenges in the Diagnosis of SARS-CoV-2 Infection in Children
Abstract
1. Introduction
Research Questions
- What is the level of preparedness of healthcare personnel for correctly applying the nasopharyngeal sampling technique by recommended protocols?
- What are the most frequent errors or protocol deviations observed in applying the sampling technique?
- How is the nasopharyngeal sampling procedure perceived in terms of discomfort or adverse reactions, and how does this perception vary by age, gender, or professional background?
- What is the level of agreement between the rapid antigen test results and those of RT-PCR in pediatric patients tested concurrently?
2. Materials and Methods
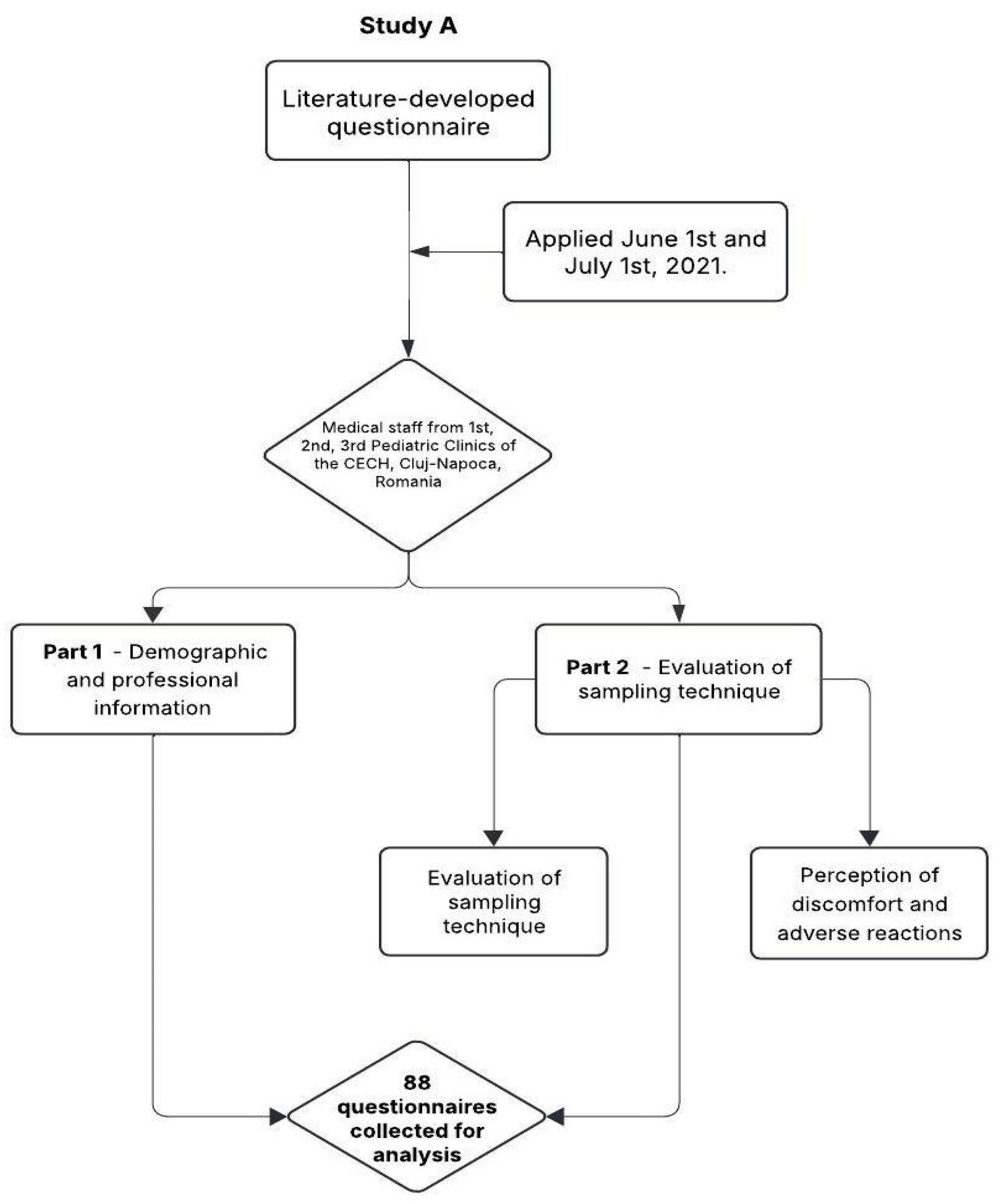
2.1. Study Design
2.2. Study A: Assessment of Medical Staff Training
2.2.1. Study Objectives
2.2.2. Questionnaire Development
2.2.3. Questionnaire Structure
- Demographic and professional information
- 2.
- Evaluation of sampling technique
- Yes—nasal hygiene (blowing the nose) was performed, implying that the participant had nasal secretions;
- No—nasal hygiene was not performed, though it remained unclear whether this was due to an omission or because it was not required;
- This was not the case—the participant did not have rhinorrhea, so this step was not applicable.
- 3.
- Perception of discomfort and adverse reactions
2.2.4. Data Collection
2.2.5. Testing Methods
2.3. Statistical Analysis
3. Results
3.1. Study A
3.1.1. Demographic and Professional Characteristics
3.1.2. Compliance with Sampling Protocol
3.1.3. Most Common Errors in Nasal Secretion Collection
3.1.4. Presence of Nasal Secretions
3.1.5. Correctness Score for Nasal Secretion Collection
3.1.6. Comparison of Scores Between Professional Categories
3.1.7. Adverse Reactions During Sampling
3.1.8. Factors Influencing Adverse Reactions
3.1.9. Influence of Profession and Education Level on Adverse Reactions
3.1.10. Correlation Between Sampling Procedure and Side Effects
3.1.11. Significant Aspects of the Procedure
3.2. Study B
- Mean age of the participants: 3.6 ± 4.5 years, ranged between 1 month and 18 years old.
- Gender distribution: 101 males (51.0%) and 97 females (49%).
- The majority of the participants came from an urban area (53.0%) (Table 4).
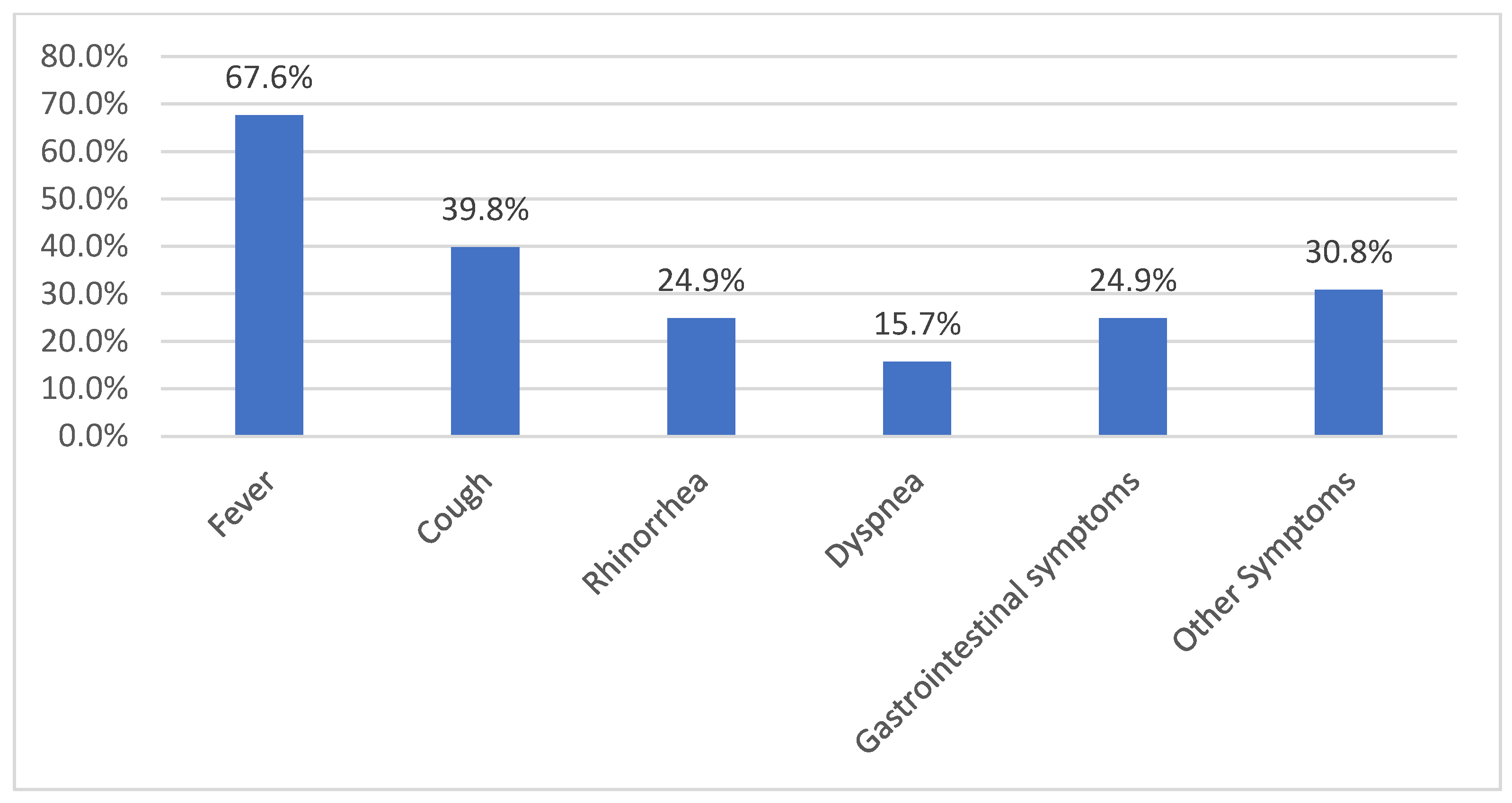
Clinical Symptoms at Admission in Study B
4. Discussion
4.1. The Assessment of the Technique for Nasopharyngeal Sampling
4.2. The Downsides of Nasopharyngeal Sampling
4.3. Diagnosis Accuracy of Rapid Antigen Test
4.4. Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Conflicts of Interest
Abbreviations
| CECH | Children’s Emergency Clinical Hospital |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| PPE | Personal protective equipment |
| CDC | Centers for Disease Control and Prevention |
| WHO | World Health Organization |
References
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.-S.; Tan, M.; Wu, S.; Chua, A.; Verma, M.; Shrestha, P.; Singh, S.; et al. Health Systems Resilience in Managing the COVID-19 Pandemic: Lessons from 28 Countries. Nat. Med. 2021, 27, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Kuo, R.-L.; Shih, S.-R. COVID-19: The First Documented Coronavirus Pandemic in History. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Netland, J. Coronaviruses Post-SARS: Update on Replication and Pathogenesis. Nat. Rev. Microbiol. 2009, 7, 439–450. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Coronavirus Envelope Protein: Current Knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef]
- Vallamkondu, J.; John, A.; Wani, W.Y.; Ramadevi, S.P.; Jella, K.K.; Reddy, P.H.; Kandimalla, R. SARS-CoV-2 Pathophysiology and Assessment of Coronaviruses in CNS Diseases with a Focus on Therapeutic Targets. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165889. [Google Scholar] [CrossRef]
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and SARS-CoV-2. Turk. J. Med. Sci. 2020, 50, 549–556. [Google Scholar] [CrossRef]
- Payne, S. Family Coronaviridae. Viruses 2017, 149–158. [Google Scholar] [CrossRef]
- Park, S.E. Epidemiology, Virology, and Clinical Features of Severe Acute Respiratory Syndrome -Coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clin. Exp. Pediatr. 2020, 63, 119–124. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Bell, K.J.; Li, Y.; Medcalf, E.; Ackermann, D. COVID-19 Rapid Antigen Tests Approved for Self-Testing in Australia: Published Diagnostic Test Accuracy Studies and Manufacturer-Supplied Information. A Systematic Review. Med. J. Aust. 2023, 219, 551–558. [Google Scholar] [CrossRef]
- Loeffelholz, M.J.; Tang, Y.-W. Laboratory Diagnosis of Emerging Human Coronavirus Infections—The State of the Art. Emerg. Microbes Infect. 2020, 9, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Heymann, D.L.; Teo, Y.-Y.; Garcia, P.J. Diagnostics for COVID-19: Moving from Pandemic Response to Control. Lancet Lond. Engl. 2022, 399, 757–768. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.-W.; Sridhar, S.; Chiu, K.H.-Y.; Hung, D.L.-L.; Li, X.; Hung, I.F.-N.; Tam, A.R.; Chung, T.W.-H.; Chan, J.F.-W.; Zhang, A.J.-X.; et al. Lessons Learned 1 Year after SARS-CoV-2 Emergence Leading to COVID-19 Pandemic. Emerg. Microbes Infect. 2021, 10, 507–535. [Google Scholar] [CrossRef] [PubMed]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 Diagnosis -A Review of Current Methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Marty, F.M.; Chen, K.; Verrill, K.A. How to Obtain a Nasopharyngeal Swab Specimen. N. Engl. J. Med. 2020, 382, e76. [Google Scholar] [CrossRef]
- CDC Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing. Available online: https://www.cdc.gov/covid/hcp/clinical-care/clinical-specimen-guidelines.html (accessed on 26 February 2025).
- World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance, 2 March 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/331329/WHO-COVID-19-laboratory-2020.4-eng.pdf (accessed on 2 March 2024).
- Fazio, E.; Abousiam, M.; Caselli, A.; Accorona, R.; Nebiaj, A.; Ermoli, I.; Erckert, B.; Calabrese, L.; Gazzini, L. Proper Procedures for Performing Nasopharyngeal and Oropharyngeal Swabs for COVID-19. ATS Sch. 2020, 1, 495–497. [Google Scholar] [CrossRef]
- Tong, J.Y.; Wong, A.; Zhu, D.; Fastenberg, J.H.; Tham, T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-Analysis. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2020, 163, 3–11. [Google Scholar] [CrossRef]
- Li, L.; Shim, T.; Zapanta, P.E. Optimization of COVID-19 Testing Accuracy with Nasal Anatomy Education. Am. J. Otolaryngol. 2021, 42, 102777. [Google Scholar] [CrossRef]
- Li, W.; Zhou, H.; Guo, Q.; Li, G. The Nasopharynx Swab Test for Coronavirus Disease-2019 Is Mild and Will Not Cause Significant Pain and Anxiety: A Cross-Sectional Study Based on Psychiatrists. Front. Cell. Infect. Microbiol. 2021, 11, 592092. [Google Scholar] [CrossRef]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender Differences in Anxiety Disorders: Prevalence, Course of Illness, Comorbidity and Burden of Illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef]
- Zajacova, A.; Jehn, A.; Stackhouse, M.; Denice, P.; Ramos, H. Changes in Health Behaviours during Early COVID-19 and Socio-Demographic Disparities: A Cross-Sectional Analysis. Can. J. Public Health 2020, 111, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Cutler, D.M.; Lleras-Muney, A. Understanding Differences in Health Behaviors by Education. J. Health Econ. 2010, 29, 1–28. [Google Scholar] [CrossRef]
- Grol-Prokopczyk, H. Sociodemographic Disparities in Chronic Pain, Based on 12-Year Longitudinal Data. Pain 2017, 158, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, Z.; Zajacova, A. Persistent, Consistent, and Extensive: The Trend of Increasing Pain Prevalence in Older Americans. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020, 75, 436–447. [Google Scholar] [CrossRef]
- Fliesser, M.; De Witt Huberts, J.; Wippert, P.-M. Education, Job Position, Income or Multidimensional Indices? Associations between Different Socioeconomic Status Indicators and Chronic Low Back Pain in a German Sample: A Longitudinal Field Study. BMJ Open 2018, 8, e020207. [Google Scholar] [CrossRef]
- Janevic, M.R.; McLaughlin, S.J.; Heapy, A.A.; Thacker, C.; Piette, J.D. Racial and Socioeconomic Disparities in Disabling Chronic Pain: Findings from the Health and Retirement Study. J. Pain 2017, 18, 1459–1467. [Google Scholar] [CrossRef]
- Newman, A.K.; Van Dyke, B.P.; Torres, C.A.; Baxter, J.W.; Eyer, J.C.; Kapoor, S.; Thorn, B.E. The Relationship of Sociodemographic and Psychological Variables with Chronic Pain Variables in a Low-Income Population. Pain 2017, 158, 1687–1696. [Google Scholar] [CrossRef]
- Bruning, A.H.L.; Leeflang, M.M.G.; Vos, J.M.B.W.; Spijker, R.; de Jong, M.D.; Wolthers, K.C.; Pajkrt, D. Rapid Tests for Influenza, Respiratory Syncytial Virus, and Other Respiratory Viruses: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 1026–1032. [Google Scholar] [CrossRef]
- Clark, T.W.; Lindsley, K.; Wigmosta, T.B.; Bhagat, A.; Hemmert, R.B.; Uyei, J.; Timbrook, T.T. Rapid Multiplex PCR for Respiratory Viruses Reduces Time to Result and Improves Clinical Care: Results of a Systematic Review and Meta-Analysis. J. Infect. 2023, 86, 462–475. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Filbin, M.R.; Hou, P.C.; Kurz, M.C.; Han, J.H.; Aufderheide, T.P.; Ward, M.A.; Pulia, M.S.; Birkhahn, R.H.; Diaz, J.L.; et al. Diagnostic Accuracy of a Bacterial and Viral Biomarker Point-of-Care Test in the Outpatient Setting. JAMA Netw. Open 2022, 5, e2234588. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Mateos-Haro, M.; Dinnes, J.; Ciapponi, A.; Davenport, C.; Buitrago-Garcia, D.; Bennouna-Dalero, T.; Roqué-Figuls, M.; Van den Bruel, A.; von Eije, K.J.; et al. Laboratory-Based Molecular Test Alternatives to RT-PCR for the Diagnosis of SARS-CoV-2 Infection. Cochrane Database Syst. Rev. 2024, 10, CD015618. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.M.; Al-Waleedi, A.A.; Al-Shaibani, S.M.; Rajamanar, M.; Al-Akbari, S.; Al-Harazi, A.; Salim Aliwah, L.; Ahmed Salem, N.; Al-Ademi, D.; Barakat, A.; et al. Strengthening Laboratories in Response to Outbreaks in Humanitarian Emergencies and Conflict Settings: Results, Challenges and Lessons from Expanding PCR Diagnostic Capacities for COVID-19 Testing in Yemen. PLoS ONE 2024, 19, e0298603. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Li, T.-D.; Zheng, S.-F.; Su, Y.-Y.; Li, Z.-Y.; Liu, W.; Yu, F.; Ge, S.-X.; Zou, Q.-D.; Yuan, Q.; et al. Serology Characteristics of SARS-CoV-2 Infection after Exposure and Post-Symptom Onset. Eur. Respir. J. 2020, 56, 2000763. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, V.A.d.F.; Gomes, J.C.; de Santana, M.A.; de Lima, C.L.; Calado, R.B.; Bertoldo Júnior, C.R.; de Albuquerque, J.E.A.; de Souza, R.G.; de Araújo, R.J.E.; Mattos Júnior, L.A.R.; et al. Covid-19 Rapid Test by Combining a Random Forest-Based Web System and Blood Tests. J. Biomol. Struct. Dyn. 2022, 40, 11948–11967. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, Point-of-Care Antigen and Molecular-Based Tests for Diagnosis of SARS-CoV-2 Infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar] [CrossRef]
- Hirabayashi, E.; Mercado, G.; Hull, B.; Soin, S.; Koshy-Chenthittayil, S.; Raman, S.; Huang, T.; Keerthisinghe, C.; Feliciano, S.; Dongo, A.; et al. Comparison of Diagnostic Accuracy of Rapid Antigen Tests for COVID-19 Compared to the Viral Genetic Test in Adults: A Systematic Review and Meta-Analysis. JBI Evid. Synth. 2024, 22, 1939–2002. [Google Scholar] [CrossRef]
- Sajal, S.S.A.; Islam, D.Z.; Khandker, S.S.; Solórzano-Ortiz, E.; Fardoun, M.; Ahmed, M.F.; Jamiruddin, M.R.; Azmuda, N.; Mehta, M.; Kumar, S.; et al. Strategies to Overcome Erroneous Outcomes in Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Testing: Insights From the COVID-19 Pandemic. Cureus 2024, 16, e72954. [Google Scholar] [CrossRef]
- Mostert, S.; Hoogland, M.; Huibers, M.; Kaspers, G. Excess Mortality across Countries in the Western World since the COVID-19 Pandemic: ‘Our World in Data’ Estimates of January 2020 to December 2022. BMJ Public Health 2024, 2, e000282. [Google Scholar] [CrossRef]

| Question | Answer | ||
|---|---|---|---|
| Yes (%) | No (%) | Do Not Know (%) | |
| Was the head slightly bent back during sample collection? | 78.4 | 21.6 | |
| Was the tip of the nose lifted? | 52.3 | 42.0 | 5.7 |
| Was the nurse positioned on the side of the nostril where the collection was performed? | 59.1 | 37.5 | 3.4 |
| Was the functional nostril established? | 41.0 | 55.6 | 3.4 |
| Was the hygiene of the nasal cavity performed before the test? | 21.6 | 4.50 | 73.9 (was not the case) |
| Was the nasal swab inserted up to the level of the posterior wall? | 93.1 | 2.30 | 4.6 |
| Was the pad rotated for 10 s? | 77.2 | 11.4 | 11.4 |
| Was the nasal swab withdrawn slowly without touching the skin? | 86.4 | 9.10 | 4.5 |
| Was a mask worn and protective equipment used by the person performing the sample collection? | 61.4 | 37.5 | 1.1 |
| Was the result of the rapid test communicated within 15 min? | 97.8 | 1.10 | 1.1 |
| Did you feel any pain during collection of nasal secretion? | 46.6 | 53.4 | |
| Did you sneeze, cough, or have other symptoms during the sample collection? | 44.3 | 55.7 | |
| Number | Percentage | p Value | |
|---|---|---|---|
| Q11. Did you feel any pain during the nasal swab collection? | |||
| Age, years | 0.064 | ||
| <41 years | 26/49 | 53.1% | |
| ≥41 years | 13/39 | 33.3% | |
| Gender | 0.074 | ||
| Male | 5/19 | 26.3% | |
| Female | 34/69 | 49.3% | |
| Profession, level of training | 0.466 | ||
| physician | 19/38 | 50.0% | |
| nurse and registered nurse | 13/29 | 44.8% | |
| other healthcare provider | 7/21 | 33.3% | |
| Q12. Did you sneeze, cough, or have other symptoms during the sample collection? | |||
| Age, years | 0.005 | ||
| <41 years | 35/49 | 71.4% | |
| ≥41 years | 37/39 | 94.9% | |
| Gender | 0.714 | ||
| males | 15/19 | 78.9% | |
| females | 57/69 | 82.6% | |
| Profession, level of training | 0.003 | ||
| physician | 25/38 | 65.8% | |
| nurse and registered nurse | 22/29 | 93.1% | |
| other healthcare provider | 20/21 | 95.2% | |
| Q11. Pain During Procedure | Q12. Sneeze, Cough, or Other Symptoms | |||||
|---|---|---|---|---|---|---|
| % Answer | % Answer | |||||
| YES versus NO | p | phi/V | YES Versus NO | p | phi/V | |
| The head was slightly bent back during sample collection | 36.7% 68.4% | 0.017 | Phi = 0.255 | 78.3% 94.7% | 0.176 * | Phi = 0.17 |
| Tip of the nose lifted | 37.0% 52.4% | 0.146 | Phi = 0.155 | 78.3% 85.7% | 0.365 | Phi = 0.09 |
| The nurse on the side of the nostril where the collection was performed | 42.3% 47.2% | 0.640 * | Phi = 0.0486 | 82.7% 80.6% | 0.790 | Phi = 0.027 |
| The functional nostril established | 50.0% 40.4% | 0.372 | Phi = 0.090 | 88.9% 76.9% | 0.152 | Phi = 0.15 |
| The hygiene of the nasal cavity before the test ** | 63.2% 75.0% 36.9% | 0.057 | V = 0.250 | 68.4% 75.0% 86.2% | 0.114 * | V = 0.192 |
| The nasal swab inserted up to the level of the posterior wall | 45.1% 33.0% | 0.689 | Phi = 0.050 | 80.5% 100% | 0.587 * | Phi = 0.12 |
| The pad rotated for 10 s | 47.1% 35.0% | 0.340 | Phi = 0.102 | 88.2% 60.0% | 0.008 * | Phi = 0.307 |
| The nasal swab was withdrawn slowly | 44.7% 41.7% | 0.842 | Phi = 0.020 | 82.9% 75.0% | 0.451 * | Phi = 0.07 |
| Parameter | Value |
|---|---|
| Age, mean ± SD | 3.6 ± 4.5 years |
| Age groups, no (%) | |
| infants, 0–12 months old | 58 patients (29.3%) |
| 1–3 years old | 83 patients (41.9%) |
| 4–6 years old | 20 patients (10.1%) |
| >7 years old | 37 patients (18.7%) |
| Males, no (%) | 101/198 (51.0%) |
| Environment, no (%) | |
| urban area | 105/198 (53.0%) |
| rural area | 78/198 (39.4%) |
| not specified | 15/198 (7.6%) |
| RT-PCR Positive | RT-PCR Negative | Total | |
|---|---|---|---|
| Rapid Test Positive | 0 | 3 | 3 |
| Rapid Test Negative | 5 | 190 | 195 |
| Total | 5 | 193 | 198 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouari-Coblișan, A.P.; Pop, C.F.; Sas, V.; Borcău, A.G.; Bonci, T.I.; Cherecheș-Panța, P. Practical Challenges in the Diagnosis of SARS-CoV-2 Infection in Children. Nurs. Rep. 2025, 15, 196. https://doi.org/10.3390/nursrep15060196
Bouari-Coblișan AP, Pop CF, Sas V, Borcău AG, Bonci TI, Cherecheș-Panța P. Practical Challenges in the Diagnosis of SARS-CoV-2 Infection in Children. Nursing Reports. 2025; 15(6):196. https://doi.org/10.3390/nursrep15060196
Chicago/Turabian StyleBouari-Coblișan, Alina Petronela, Claudia Felicia Pop, Valentina Sas, Adina Georgiana Borcău, Teodora Irina Bonci, and Paraschiva Cherecheș-Panța. 2025. "Practical Challenges in the Diagnosis of SARS-CoV-2 Infection in Children" Nursing Reports 15, no. 6: 196. https://doi.org/10.3390/nursrep15060196
APA StyleBouari-Coblișan, A. P., Pop, C. F., Sas, V., Borcău, A. G., Bonci, T. I., & Cherecheș-Panța, P. (2025). Practical Challenges in the Diagnosis of SARS-CoV-2 Infection in Children. Nursing Reports, 15(6), 196. https://doi.org/10.3390/nursrep15060196







