Influenza Vaccination of Nurses and Other Health Care Workers in Different Occupational Settings: A Classic and AI Mixed Approach for Time-to-Event Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Aim
2.2. Study Setting and Timeline
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.4.1. Classification of Health Care Workers
2.4.2. Classification of the Hospital Units
2.5. Data Analysis—Classic Statistics and Cox Model
2.6. Data Analysis—AI Model for Variable Importance Assessment
2.7. Ethical Considerations
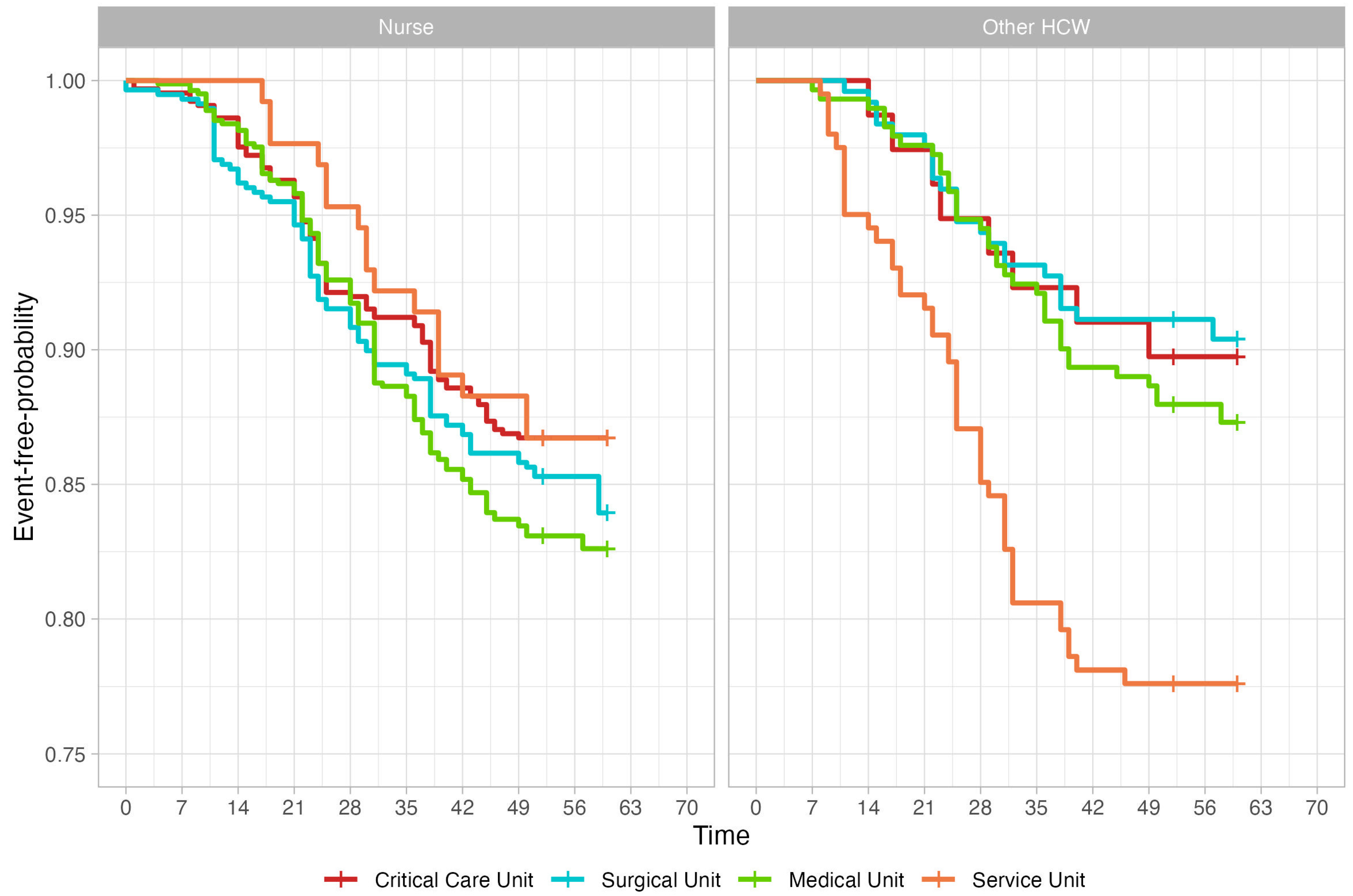
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Acknowledgments
Conflicts of Interest
Abbreviations
| HCW | Health care worker |
| AI/ML | Artificial intelligence/machine learning |
| WHO | World Health Organization |
| ECDC | European Centre for Disease Prevention and Control |
| EU | European Union |
| CDC | Centers of Disease Control and Prevention |
| ILI | Influenza-Like Illness |
References
- European Centre for Disease Prevention and Control. Factsheet About Seasonal Influenza—ecdc.europa.eu. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet#the-pathogen (accessed on 17 December 2024).
- World Health Organization (WHO). Vaccines against influenza: WHO position paper. Wkly. Epidemiol. Rec. 2022, 97, 185–208. [Google Scholar]
- Kuster, S.P.; Shah, P.S.; Coleman, B.L.; Lam, P.P.; Tong, A.; Wormsbecker, A.; McGeer, A. Incidence of influenza in healthy adults and healthcare workers: A systematic review and meta-analysis. PLoS ONE 2011, 6, e26239. [Google Scholar] [CrossRef] [PubMed]
- Bénet, T.; Amour, S.; Valette, M.; Saadatian-Elahi, M.; Aho-Glélé, L.S.; Berthelot, P.; Denis, M.A.; Grando, J.; Landelle, C.; Astruc, K.; et al. Incidence of asymptomatic and symptomatic influenza among healthcare workers: A multicenter prospective cohort study. Clin. Infect. Dis. 2021, 72, e311–e318. [Google Scholar] [CrossRef]
- Tokars, J.I.; Olsen, S.J.; Reed, C. Seasonal incidence of symptomatic influenza in the United States. Clin. Infect. Dis. 2018, 66, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Amodio, E.; Restivo, V.; Firenze, A.; Mammina, C.; Tramuto, F.; Vitale, F. Can influenza vaccination coverage among healthcare workers influence the risk of nosocomial influenza-like illness in hospitalized patients? J. Hosp. Infect. 2014, 86, 182–187. [Google Scholar] [CrossRef]
- Bénet, T.; Régis, C.; Voirin, N.; Robert, O.; Lina, B.; Cronenberger, S.; Comte, B.; Coppéré, B.; Vanhems, P. Influenza vaccination of healthcare workers in acute-care hospitals: A case-control study of its effect on hospital-acquired influenza among patients. BMC Infect. Dis. 2012, 12, 30. [Google Scholar] [CrossRef]
- Li, T.; Qi, X.; Li, Q.; Tang, W.; Su, K.; Jia, M.; Yang, W.; Xia, Y.; Xiong, Y.; Qi, L.; et al. A systematic review and meta-analysis of seasonal influenza vaccination of health workers. Vaccines 2021, 9, 1104. [Google Scholar] [CrossRef]
- Ratti, M.; Concina, D.; Rinaldi, M.; Salinelli, E.; Di Brisco, A.M.; Ferrante, D.; Volpe, A.; Panella, M. Vaccination strategies against seasonal influenza in long term care setting: Lessons from a mathematical modelling study. Vaccines 2022, 11, 32. [Google Scholar] [CrossRef]
- van den Dool, C.; Bonten, M.J.M.; Hak, E.; Wallinga, J. Modeling the effects of influenza vaccination of health care workers in hospital departments. Vaccine 2009, 27, 6261–6267. [Google Scholar] [CrossRef]
- Dos Santos, G.; Neumeier, E.; Bekkat-Berkani, R. Influenza: Can we cope better with the unpredictable? Hum. Vaccin. Immunother. 2016, 12, 699–708. [Google Scholar] [CrossRef]
- Nsoesie, E.O.; Brownstein, J.S.; Ramakrishnan, N.; Marathe, M.V. A systematic review of studies on forecasting the dynamics of influenza outbreaks. Influenza Other Respi. Viruses 2014, 8, 309–316. [Google Scholar] [CrossRef] [PubMed]
- CDC - Centers for Disease Control and prevention. About Flu Forecasting—cdc.gov. Available online: https://www.cdc.gov/flu-forecasting/about/index.html (accessed on 20 December 2024).
- Ferdinands, J.M.; Alyanak, E.; Reed, C.; Fry, A.M. Waning of influenza vaccine protection: Exploring the trade-offs of changes in vaccination timing among older adults. Clin. Infect. Dis. 2020, 70, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Murti, M.; Otterstatter, M.; Orth, A.; Balshaw, R.; Halani, K.; Brown, P.D.; Hejazi, S.; Thompson, D.; Allison, S.; Bharmal, A.; et al. Measuring the impact of influenza vaccination on healthcare worker absenteeism in the context of a province-wide mandatory vaccinate-or-mask policy. Vaccine 2019, 37, 4001–4007. [Google Scholar] [CrossRef] [PubMed]
- Saadeh-Navarro, E.; Garza-González, E.; Salazar-Montalvo, R.G.; Rodríguez-López, J.M.; Mendoza-Flores, L.; Camacho-Ortiz, A. Association between early influenza vaccination and the reduction of influenza-like syndromes in health care providers. Am. J. Infect. Control 2016, 44, 250–252. [Google Scholar] [CrossRef]
- Tian, C.; Lovrics, O.; Vaisman, A.; Chin, K.J.; Tomlinson, G.; Lee, Y.; Englesakis, M.; Parotto, M.; Singh, M. Risk factors and protective measures for healthcare worker infection during highly infectious viral respiratory epidemics: A systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2022, 43, 639–650. [Google Scholar] [CrossRef]
- Torén, K.; Albin, M.; Bergström, T.; Alderling, M.; Schioler, L.; Åberg, M. Occupational risks for infection with influenza A and B: A national case-control study covering 1 July 2006–31 December 2019. Occup. Environ. Med. 2023, 80, 377–383. [Google Scholar] [CrossRef]
- Macintyre, C.R.; Seale, H.; Yang, P.; Zhang, Y.; Shi, W.; Almatroudi, A.; Moa, A.; Wang, X.; Li, X.; Pang, X.; et al. Quantifying the risk of respiratory infection in healthcare workers performing high-risk procedures. Epidemiol. Infect. 2014, 142, 1802–1808. [Google Scholar] [CrossRef]
- Kormuth, K.A.; Lin, K.; Prussin, A.J., 2nd; Vejerano, E.P.; Tiwari, A.J.; Cox, S.S.; Myerburg, M.M.; Lakdawala, S.S.; Marr, L.C. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity. J. Infect. Dis. 2018, 218, 739–747. [Google Scholar] [CrossRef]
- Young, S.; Goldin, S.; Dumolard, L.; Shendale, S.; McMurren, B.; Maltezou, H.C.; Desai, S. National vaccination policies for health workers—A cross-sectional global overview. Vaccine 2024, 42, 757–769. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Piano Nazionale Prevenzione Vaccinale 2023–2025 (National Preventive Vaccination Plan 2023–2025). Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=95963&completo=true (accessed on 1 February 2025).
- Ministero dell’Università; e della Ricerca (Ministry of University and Research)—Scuole di Specializzazione (Schools of Medical Specialization). Available online: https://www.mur.gov.it/it/aree-tematiche/universita/lofferta-formativa-titoli-rilasciati/scuole-di-specializzazione (accessed on 10 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC.: Boston, MA, USA, 2020. [Google Scholar]
- Longato, E.; Vettoretti, M.; Di Camillo, B. A practical perspective on the concordance index for the evaluation and selection of prognostic time-to-event models. J. Biomed. Inform. 2020, 108, 103496. [Google Scholar] [CrossRef]
- Lambert, J.; Chevret, S. Summary measure of discrimination in survival models based on cumulative/dynamic time-dependent ROC curves. Stat. Methods Med. Res. 2016, 25, 2088–2102. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.E.; Kim, H.; Park, S.H. Review of statistical methods for evaluating the performance of survival or other time-to-event prediction models (from conventional to deep learning approaches). Korean J. Radiol. 2021, 22, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Spytek, M.; Krzyziński, M.; Langbein, S.H.; Baniecki, H.; Wright, M.N.; Biecek, P. survex: An R package for explaining machine learning survival models. Bioinformatics 2023, 39, btad723. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.N.; Ziegler, A. Ranger: A fast implementation of random forests for high dimensional data in C++ and R. J. Stat. Softw. 2017, 77, 1–17. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021-22 influenza season. MMWR Recomm. Rep. 2021, 70, 1–28. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Tafuri, S.; Spinelli, G.; Carlucci, M.; Migliore, G.; Calabrese, G.; Daleno, A.; Melpignano, L.; Vimercati, L.; Stefanizzi, P. Two years of on-site influenza vaccination strategy in an Italian university hospital: Main results and lessons learned. Hum. Vaccin. Immunother. 2022, 18, 1993039. [Google Scholar] [CrossRef]
- Minardi, V.; Gallo, R.; Possenti, V.; Contoli, B.; Di Fonzo, D.; D’Andrea, E.; Masocco, M. Influenza vaccination uptake and prognostic factors among health professionals in Italy: Results from the nationwide surveillance PASSI 2015–2018. Vaccines 2023, 11, 1223. [Google Scholar] [CrossRef]
- Domnich, A.; Cambiaggi, M.; Vasco, A.; Maraniello, L.; Ansaldi, F.; Baldo, V.; Bonanni, P.; Calabrò, G.E.; Costantino, C.; de Waure, C.; et al. Attitudes and beliefs on influenza vaccination during the COVID-19 pandemic: Results from a representative Italian survey. Vaccines 2020, 8, 711. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Cuscianna, E.; Rizzi, D.; Signorile, N.; Daleno, A.; Migliore, G.; Tafuri, S. Impact of COVID-19 pandemic on flu vaccine uptake in healthcare workers in Europe: A systematic review and meta-analysis. Expert Rev. Vaccines 2023, 22, 777–784. [Google Scholar] [CrossRef]
- Chen, R.; Gilbert, N.L.; Dubé, È. Adult influenza vaccination coverage before, during and after the COVID-19 pandemic in Canada. BMC Public Health 2024, 24, 3357. [Google Scholar] [CrossRef]
- Caban-Martinez, A.J.; Lee, D.J.; Davila, E.P.; LeBlanc, W.G.; Arheart, K.L.; McCollister, K.E.; Christ, S.L.; Clarke, T.; Fleming, L.E. Sustained low influenza vaccination rates in US healthcare workers. Prev. Med. 2010, 50, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Neufeind, J.; Wenchel, R.; Boedeker, B.; Wicker, S.; Wichmann, O. Monitoring influenza vaccination coverage and acceptance among health-care workers in German hospitals—Results from three seasons. Hum. Vaccin. Immunother. 2021, 17, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.; Wu, C.T.S.; Le-Nguyen, A.F.; Czock, A. Influenza vaccination behaviour of healthcare workers in Switzerland: A cross-sectional study. Int. J. Public Health 2023, 68, 1605175. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, E.; Jansen, A.; Lopalco, P.L.; Giesecke, J. Ethics of mandatory vaccination for healthcare workers. Euro Surveill. 2013, 18, 20627. [Google Scholar] [CrossRef]
- Van Hooste, W.L.C.; Bekaert, M. To be or not to be vaccinated? The ethical aspects of influenza vaccination among healthcare workers. Int. J. Environ. Res. Public Health 2019, 16, 3981. [Google Scholar] [CrossRef]
- Simberkoff, M.S.; Rattigan, S.M.; Gaydos, C.A.; Gibert, C.L.; Gorse, G.J.; Nyquist, A.C.; Price, C.S.; Reich, N.; Rodriguez-Barradas, M.C.; Bessesen, M.; et al. Impact of mandatory vaccination of healthcare personnel on rates of influenza and other viral respiratory pathogens. Infect. Control Hosp. Epidemiol. 2022, 43, 1216–1220. [Google Scholar] [CrossRef]
- Lytras, T.; Kopsachilis, F.; Mouratidou, E.; Papamichail, D.; Bonovas, S. Interventions to increase seasonal influenza vaccine coverage in healthcare workers: A systematic review and meta-regression analysis. Hum. Vaccin. Immunother. 2016, 12, 671–681. [Google Scholar] [CrossRef]
- Kitt, E.; Burt, S.; Price, S.M.; Satchell, L.; Offit, P.A.; Sammons, J.S.; Coffin, S.E. Implementation of a mandatory influenza vaccine policy: A 10-year experience. Clin. Infect. Dis. 2021, 73, e290–e296. [Google Scholar] [CrossRef]
- Durovic, A.; Widmer, A.F.; Dangel, M.; Ulrich, A.; Battegay, M.; Tschudin-Sutter, S. Low rates of influenza vaccination uptake among healthcare workers: Distinguishing barriers between occupational groups. Am. J. Infect. Control 2020, 48, 1139–1143. [Google Scholar] [CrossRef]
- Vimercati, L.; Bianchi, F.P.; Mansi, F.; Ranieri, B.; Stefanizzi, P.; De Nitto, S.; Tafuri, S. Influenza vaccination in health-care workers: An evaluation of an on-site vaccination strategy to increase vaccination uptake in HCWs of a South Italy Hospital. Hum. Vaccin. Immunother. 2019, 15, 2927–2932. [Google Scholar] [CrossRef]
- Dettori, M.; Arghittu, A.; Deiana, G.; Azara, A.; Masia, M.D.; Palmieri, A.; Spano, A.L.; Serra, A.; Castiglia, P. Influenza vaccination strategies in healthcare workers: A cohort study (2018–2021) in an Italian university hospital. Vaccines 2021, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- English, K.M.; Langley, J.M.; McGeer, A.; Hupert, N.; Tellier, R.; Henry, B.; Halperin, S.A.; Johnston, L.; Pourbohloul, B. Contact among healthcare workers in the hospital setting: Developing the evidence base for innovative approaches to infection control. BMC Infect. Dis. 2018, 18, 184. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Hyman, S.; Rosenberg, L.; Larson, E. Frequency of patient contact with health care personnel and visitors: Implications for infection prevention. Jt. Comm. J. Qual. Patient Saf. 2012, 38, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Yang, J.; Zhang, N.; Rong, W.; Gao, L.; Xia, P.; Zou, J.; Zhu, N.; Yang, F.; Chen, L. Comparison and use of explainable machine learning-based survival models for heart failure patients. Digit. Health 2024, 10, 20552076241277027. [Google Scholar] [CrossRef]
- World Health Organization. Implementation Guide for Vaccination of Health Workers; World Health Organization: Geneva, Switzerland, 2022; pp. 10–19. [Google Scholar]


| Levels | Overall | 2022 | 2023 | p | |
|---|---|---|---|---|---|
| n | 2982 | 1444 | 1538 | ||
| HCW Typology n (%) | Nurse | 2164 (72.6) | 1061 (73.5) | 1103 (71.7) | 0.3 |
| Other HCW | 818 (27.4) | 383 (26.5) | 435 (28.3) | ||
| Gender n (%) | Female | 2426 (81.4) | 1175 (81.4) | 1251 (81.3) | 1 |
| Male | 556 (18.6) | 269 (18.6) | 287 (18.7) | ||
| Age Category n (%) | [25, 46] | 1333 (44.7) | 629 (43.6) | 704 (45.8) | 0.239 |
| (46, 65] | 1649 (55.3) | 815 (56.4) | 834 (54.2) | ||
| Ward Typology n (%) | Critical Care Unit | 726 (24.3) | 355 (24.6) | 371 (24.1) | 0.929 |
| Surgical Unit | 826 (27.7) | 396 (27.4) | 430 (28.0) | ||
| Medical Unit | 1101 (36.9) | 538 (37.3) | 563 (36.6) | ||
| Service Unit | 329 (11.0) | 155 (10.7) | 174 (11.3) |
| Not Vaccinated | Vaccinated | p | ||
|---|---|---|---|---|
| n | 2539 | 443 | ||
| HCW Typology n (%) | Nurse | 1833 (72.2) | 331 (74.7) | 0.298 |
| Other HCW | 706 (27.8) | 112 (25.3) | ||
| Gender n (%) | Female | 2051 (80.8) | 375 (84.7) | 0.062 |
| Male | 488 (19.2) | 68 (15.3) | ||
| Age Category n (%) | [25, 46] | 1188 (46.8) | 145 (32.7) | <0.001 |
| (46,65] | 1351 (53.2) | 298 (67.3) | ||
| Ward Typology n (%) | Critical Care Unit | 632 (24.9) | 94 (21.2) | 0.041 |
| Surgical Unit | 714 (28.1) | 112 (25.3) | ||
| Medical Unit | 926 (36.5) | 175 (39.5) | ||
| Service Unit | 267 (10.5) | 62 (14.0) |
| Critical Care Unit | Surgical Unit | Medical Unit | Service Unit | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Levels | Nurses | Other HCWs | Nurses | Other HCWs | Nurses | Other HCWs | Nurses | Other HCWs | p | |
| n | 648 | 78 | 578 | 248 | 810 | 291 | 128 | 201 | ||
| Vaccinated n (%) | yes | 86 (13.3) | 8 (10.3) | 89 (15.4) | 23 (9.3) | 139 (17.2) | 36 (12.4) | 17 (13.3) | 45 (22.4) | 0.002 |
| no | 562 (86.7) | 70 (89.7) | 489 (84.6) | 225 (90.7) | 671 (82.8) | 255 (87.6) | 111 (86.7) | 156 (77.6) | ||
| Time to vaccination (median [IQR]) | 24.00 [18.00, 38.00] | 26.00 [20.75, 34.00] | 24.00 [15.00, 38.00] | 25.00 [21.50, 33.50] | 29.00 [22.00, 37.00] | 29.50 [23.00, 38.00] | 30.00 [25.00, 39.00] | 25.00 [15.00, 31.00] | – | |
| Model | C-Index 1 | Integrated C/D AUC 1 | Integrated Brier Score 2 |
|---|---|---|---|
| Cox PH | 0.5550325 | 0.5452379 | 0.1193034 |
| Random F | 0.5819838 | 0.6399568 | 0.1176841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratti, M.; Rescinito, R.; Gigante, D.; Lontano, A.; Panella, M. Influenza Vaccination of Nurses and Other Health Care Workers in Different Occupational Settings: A Classic and AI Mixed Approach for Time-to-Event Data. Nurs. Rep. 2025, 15, 87. https://doi.org/10.3390/nursrep15030087
Ratti M, Rescinito R, Gigante D, Lontano A, Panella M. Influenza Vaccination of Nurses and Other Health Care Workers in Different Occupational Settings: A Classic and AI Mixed Approach for Time-to-Event Data. Nursing Reports. 2025; 15(3):87. https://doi.org/10.3390/nursrep15030087
Chicago/Turabian StyleRatti, Matteo, Riccardo Rescinito, Domenico Gigante, Alberto Lontano, and Massimiliano Panella. 2025. "Influenza Vaccination of Nurses and Other Health Care Workers in Different Occupational Settings: A Classic and AI Mixed Approach for Time-to-Event Data" Nursing Reports 15, no. 3: 87. https://doi.org/10.3390/nursrep15030087
APA StyleRatti, M., Rescinito, R., Gigante, D., Lontano, A., & Panella, M. (2025). Influenza Vaccination of Nurses and Other Health Care Workers in Different Occupational Settings: A Classic and AI Mixed Approach for Time-to-Event Data. Nursing Reports, 15(3), 87. https://doi.org/10.3390/nursrep15030087







