Chronic Illness Perceptions and Cardiovascular Disease Risk Behaviors in Black and Latinx Sexual Minority Men with HIV: A Cross-Sectional Analysis
Abstract
1. Introduction
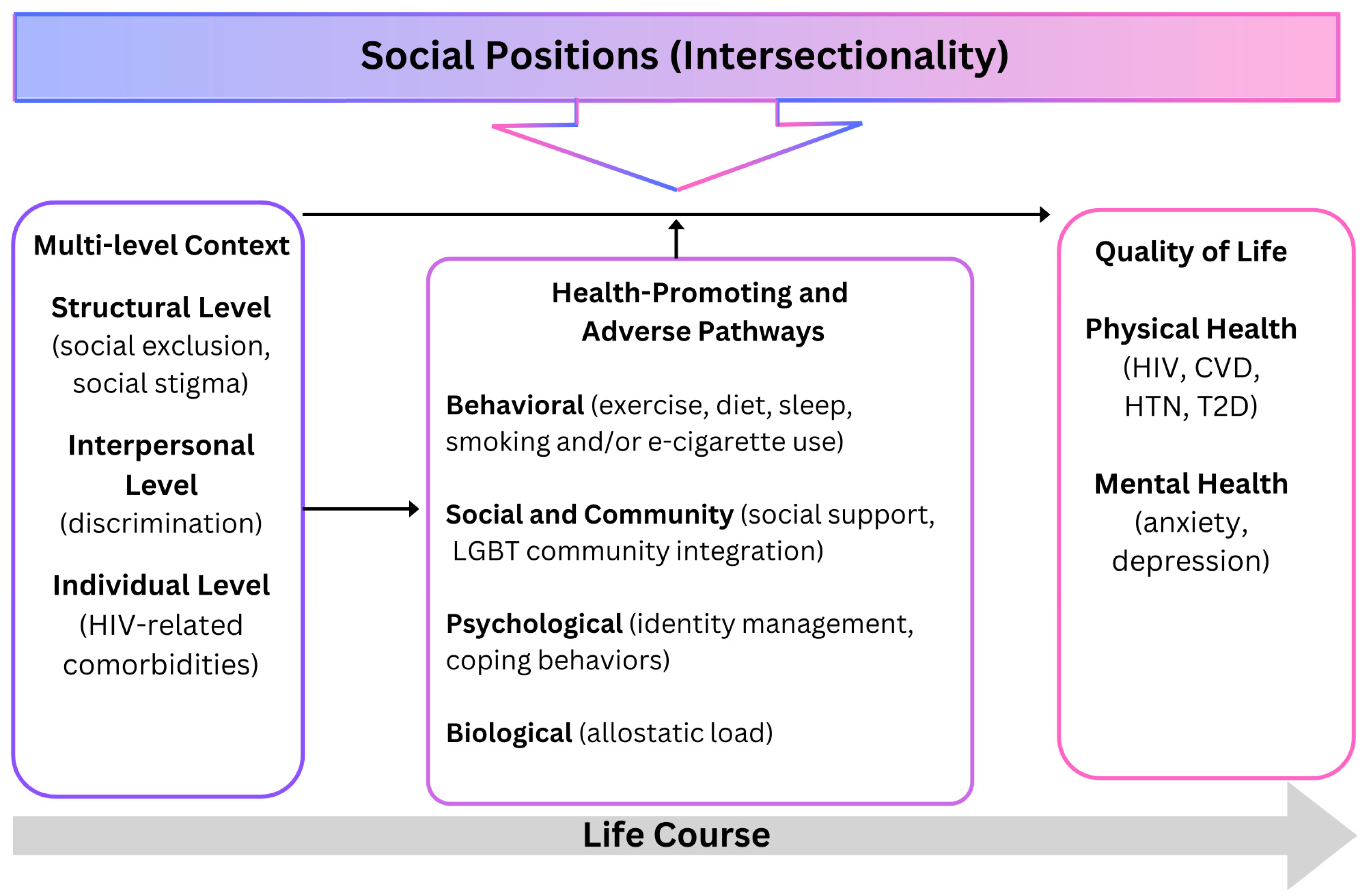
Framework
2. Materials and Methods
2.1. Study Design
2.2. Recruitment
2.3. Measures
2.3.1. Illness Perception Questionnaire-Revised
2.3.2. International Physical Activity Questionnaire
2.3.3. Behavioral Risk Factor Surveillance System
2.4. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Views on Living with Chronic Conditions
3.3. Chronic and Cyclical Timeline
3.4. Consequences
3.5. Personal Control
3.6. Treatment Control
3.7. Illness Coherence
3.8. Emotional Representations
3.9. Symptoms Related to HIV and Hypertension
3.10. Sleep Difficulties as a Primary Symptom
3.11. Physical Activity, Tobacco, and E-Cigarette Use
4. Discussion
4.1. Perceptions about Chronic Illness
4.2. Financial Toxicity
4.3. Sleep and Mental Health
4.4. Physical and Sedentary Activity and Nicotine Use
5. Limitations
6. Implications for Research and Clinical Nursing Practice
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Use of Artificial Intelligence
Acknowledgments
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Gibbs, B.B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.L.; Shah, N.S.; Lloyd-Jones, D.M.; Khan, S.S. State of the Nation’s Cardiovascular Health and Targeting Health Equity in the United States A Narrative Review. JAMA Cardiol. 2021, 6, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Hsue, P.Y.; Waters, D.D. Time to Recognize HIV Infection as a Major Cardiovascular Risk Factor. Circulation 2018, 138, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Titanji, B.; Gavegnano, C.; Hsue, P.; Schinazi, R.; Marconi, V.C. Targeting Inflammation to Reduce Atherosclerotic Cardiovascular Risk in People With HIV Infection. J. Am. Heart Assoc. 2020, 9, e014873. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association News. As HIV Patients Live Longer, Heart Disease Might Be Their Next Challenge. Available online: https://www.heart.org/en/news/2019/06/03/as-hiv-patients-live-longer-heart-disease-might-be-their-next-challenge (accessed on 22 October 2022).
- Feinstein, M.J.; Hsue, P.Y.; Benjamin, L.A.; Bloomfield, G.S.; Currier, J.S.; Freiberg, M.S.; Grinspoon, S.K.; Levin, J.; Longenecker, C.T.; Post, W.S. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation 2019, 140, E98–E124. [Google Scholar] [CrossRef] [PubMed]
- Gosiker, B.J.; Lesko, C.R.; Rich, A.J.; Crane, H.M.; Kitahata, M.M.; Reisner, S.L.; Mayer, K.H.; Fredericksen, R.J.; Chander, G.; Mathews, W.C.; et al. Cardiovascular disease risk among transgender women living with HIV in the United States. PLoS ONE 2020, 15, e0236177. [Google Scholar] [CrossRef] [PubMed]
- Dau, B.; Holodniy, M. The Relationship Between HIV Infection and Cardiovascular Disease. Curr. Cardiol. Rev. 2008, 4, 203–218. [Google Scholar] [CrossRef]
- Bygrave, H.; Golob, L.; Wilkinson, L.; Roberts, T.; Grimsrud, A. Let’s talk chronic disease: Can differentiated service delivery address the syndemics of HIV, hypertension and diabetes? Curr. Opin. HIV AIDS 2020, 15, 256–260. [Google Scholar] [CrossRef]
- Losina, E.; Hyle, E.P.; Borre, E.D.; Linas, B.P.; E Sax, P.; Weinstein, M.C.; Rusu, C.; Ciaranello, A.L.; Walensky, R.P.; A Freedberg, K. Projecting 10-year, 20-year, and Lifetime Risks of Cardiovascular Disease in Persons Living With Human Immunodeficiency Virus in the United States. Clin. Infect. Dis. 2017, 65, 1266–1271. [Google Scholar] [CrossRef]
- Shah, A.S.; Stelzle, D.; Lee, K.K.; Beck, E.J.; Alam, S.; Clifford, S.; Longenecker, C.T.; Strachan, F.; Bagchi, S.; Whiteley, W.; et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation 2018, 138, 1100–1112. [Google Scholar] [CrossRef]
- Smit, M.; Brinkman, K.; Geerlings, S.; Smit, C.; Thyagarajan, K.; van Sighem, A.; de Wolf, F.; Hallett, T.B. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect. Dis. 2015, 15, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Caceres, B.A.; Ancheta, A.J.; Dorsen, C.; Newlin-Lew, K.; Edmondson, D.; Hughes, T.L. A population-based study of the intersection of sexual identity and race/ethnicity on physiological risk factors for CVD among U.S. adults (ages 18–59). Ethn. Health 2022, 27, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Caceres, B.A.; Streed, C.G.S., Jr.; Corliss, H.L.; Lloyd-Jones, D.M.; Matthews, P.A.; Mukherjee, M.; Poteat, T.; Rosendale, N.; Ross, L.M.; On behalf of the American Heart Association Council on Cardiovascular; et al. Assessing and Addressing Cardiovascular Health in LGBTQ Adults: A Scientific Statement From the American Heart Association. Circulation 2020, 142, E321–E332. [Google Scholar] [CrossRef] [PubMed]
- Rosati, F.; Williams, D.P.; Juster, R.-P.; Thayer, J.F.; Ottaviani, C.; Baiocco, R. The Cardiovascular Conundrum in Ethnic and Sexual Minorities: A Potential Biomarker of Constant Coping With Discrimination. Front. Neurosci. 2021, 15, 619171. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Carnevali, L.; Sgoifo, A.; Williams, D.P. Angry in America: Psychophysiological Responses to Unfair Treatment. Ann. Behav. Med. 2020, 54, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Streed, C.G.; Beach, L.B.; Caceres, B.A.; Dowshen, N.L.; Moreau, K.L.; Mukherjee, M.; Poteat, T.; Radix, A.; Reisner, S.L.; Singh, V.; et al. Assessing and Addressing Cardiovascular Health in People Who Are Transgender and Gender Diverse: A Scientific Statement From the American Heart Association. Circulation 2021, 144, E136–E148. [Google Scholar] [CrossRef] [PubMed]
- Commodore-Mensah, Y.; Loustalot, F.; Himmelfarb, C.D.; Desvigne-Nickens, P.; Sachdev, V.; Bibbins-Domingo, K.; Clauser, S.B.; Cohen, D.J.; Egan, B.M.; Fendrick, A.M.; et al. Proceedings From a National Heart, Lung, and Blood Institute and the Centers for Disease Control and Prevention Workshop to Control Hypertension. Am. J. Hypertens. 2022, 35, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation 2022, 146, E18–E43. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.R.; Warren, R.; Shedlin, M.; Melkus, G.; Kershaw, T.; Vorderstrasse, A. A Framework for Using eHealth Interventions to Overcome Medical Mistrust Among Sexual Minority Men of Color Living with Chronic Conditions. Behav. Med. 2019, 45, 166–176. [Google Scholar] [CrossRef]
- Agarwala, A.; Patel, J.; Stephens, J.; Roberson, S.; Scott, J.; Beckie, T.; Jackson, E.A.; on behalf of the American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention and Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Lifestyle and Cardiometabolic Health; et al. Implementation of Prevention Science to Eliminate Health Care Inequities in Achieving Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 1183–1193. [Google Scholar] [CrossRef]
- Fredriksen-Goldsen, K.I.; Simoni, J.M.; Kim, H.-J.; Lehavot, K.; Walters, K.L.; Yang, J.; Hoy-Ellis, C.P.; Muraco, A. The health equity promotion model: Reconceptualization of lesbian, gay, bisexual, and transgender (LGBT) health disparities. Am. J. Orthopsychiatry 2014, 84, 653–663. [Google Scholar] [CrossRef]
- Ramos, S.R.; Fraser, M.; Araya, F.; Kim, H.Y.; Parrilla, J.A.S.; Sy, K.M.; Nagpal, R.T.; Camacho-Rivera, M.; Boutjdir, M. Community-Engaged Intervention Mapping for Cardiovascular Disease Prevention in Black and Latinx Sexual Minority Men With HIV in New York City: Protocol for a Web-Based Mixed Methods Study. JMIR Res. Protoc. 2022, 11, e41602. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Fox, S.; Duggan, M.; Rainie, L.; Purcell, K. The Diagnosis Difference: A Portrait of the 45% of US Adults Living with Chronic Health Conditions; Pew Internet & American Life Project: Washington, DC, USA, 2013. [Google Scholar]
- Moss-Morris, R.; Weinman, J.; Petrie, K.; Horne, R.; Cameron, L.; Buick, D. The Revised Illness Perception Questionnaire (IPQ-R). Psychol. Health 2002, 17, 1–16. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Pierannunzi, C.; Hu, S.S.; Balluz, L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004–2011. BMC Med Res. Methodol. 2013, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Yore, M.M.; Ham, S.A.; Ainsworth, B.E.; Kruger, J.; Reis, J.P.; Kohl, H.W.; Macera, C.A. Reliability and Validity of the Instrument Used in BRFSS to Assess Physical Activity. Med. Sci. Sports Exerc. 2007, 39, 1267–1274. [Google Scholar] [CrossRef]
- Office of Management and Budget. Provisional Guidance on the Implementation of the 1997 Standards for Federal Data on Race and Ethnicity. Available online: https://www.federalregister.gov/documents/2001/01/16/01-1132/provisional-guidance-on-the-implementation-of-the-1997-standards-for-federal-data-on-race-and (accessed on 8 January 2024).
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Ross, S.; Walker, A.; MacLeod, M.J. Patient compliance in hypertension: Role of illness perceptions and treatment beliefs. J. Hum. Hypertens. 2004, 18, 607–613. [Google Scholar] [CrossRef]
- Khera, R.; Kondamudi, N.; Liu, M.; Ayers, C.; Spatz, E.S.; Rao, S.; Essien, U.R.; Powell-Wiley, T.M.; Nasir, K.; Das, S.R.; et al. Lifetime healthcare expenses across demographic and cardiovascular risk groups: The application of a novel modeling strategy in a large multiethnic cohort study. Am. J. Prev. Cardiol. 2023, 14, 100493. [Google Scholar] [CrossRef]
- Department of Health and Human Services. Annual Update of the HHS Poverty Guidelines. Available online: https://www.federalregister.gov/documents/2023/01/19/2023-00885/annual-update-of-the-hhs-poverty-guidelines (accessed on 24 September 2023).
- New York State Department of Health. 2023–2024 Federal Income Guidelines. Available online: https://www.health.ny.gov/prevention/nutrition/wic/income_guidelines.htm (accessed on 24 September 2023).
- Scott, A.J.; Webb, T.L.; James, M.M.-S.; Rowse, G.; Weich, S. Improving sleep quality leads to better mental health: A meta-analysis of randomised controlled trials. Sleep Med. Rev. 2021, 60, 101556. [Google Scholar] [CrossRef]
- Luyster, F.S.; Strollo, P.J.; Zee, P.C.; Walsh, J.K. Sleep: A Health Imperative. Sleep 2012, 35, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Chien, W.; Chung, C.; Wu, F. Risk of type 2 diabetes in patients with insomnia: A population-based historical cohort study. Diabetes/Metabolism Res. Rev. 2018, 34, e2930. [Google Scholar] [CrossRef] [PubMed]
- Adjaye-Gbewonyo, D.; Ng, A.E.; Black, L.I. Sleep Difficulties in Adults: United States, 2020. Available online: https://stacks.cdc.gov/view/cdc/117490 (accessed on 24 September 2023).
- Ramos, S.R.; Gaffey, A.S.R.; Kang, B.; McCall, T. How Sleep Affects Mind, Body, and Heart Health. Available online: https://www.sbm.org/healthy-living/how-sleep-affects-mind-body-and-heart-health (accessed on 24 September 2023).
- Williams, N.D.; Fish, J.N. The availability of LGBT-specific mental health and substance abuse treatment in the United States. Health Serv. Res. 2020, 55, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Statista. Mental Health Facilities Offering Programs for Specific Client Groups U.S. 2020. Percentage of U.S. Available online: https://www.statista.com/statistics/712859/mental-health-facilities-offering-treatment-programs-for-specific-client-groups-in-us/ (accessed on 25 April 2024).
- Keefe, J.R.; Rodriguez-Seijas, C.; Jackson, S.D.; Bränström, R.; Harkness, A.; Safren, S.A.; Hatzenbuehler, M.L.; Pachankis, J.E. Moderators of LGBQ-affirmative cognitive behavioral therapy: ESTEEM is especially effective among Black and Latino sexual minority men. J. Consult. Clin. Psychol. 2023, 91, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Pachankis, J.E.; Harkness, A.; Maciejewski, K.R.; Behari, K.; Clark, K.A.; McConocha, E.; Winston, R.; Adeyinka, O.; Reynolds, J.; Bränström, R.; et al. LGBQ-affirmative cognitive-behavioral therapy for young gay and bisexual men’s mental and sexual health: A three-arm randomized controlled trial. J. Consult. Clin. Psychol. 2022, 90, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Kannampallil, T.; Ronneberg, C.R.; E Wittels, N.; Kumar, V.; Lv, N.; Smyth, J.M.; Gerber, B.S.; A Kringle, E.; A Johnson, J.; Yu, P.; et al. Design and Formative Evaluation of a Virtual Voice-Based Coach for Problem-solving Treatment: Observational Study. JMIR Form. Res. 2022, 6, e38092. [Google Scholar] [CrossRef] [PubMed]
- Kannampallil, T.; Ajilore, O.A.; Lv, N.; Smyth, J.M.; Wittels, N.E.; Ronneberg, C.R.; Kumar, V.; Xiao, L.; Dosala, S.; Barve, A.; et al. Effects of a virtual voice-based coach delivering problem-solving treatment on emotional distress and brain function: A pilot RCT in depression and anxiety. Transl. Psychiatry 2023, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Merschel, M. Heart Health Report Aims to Bolster Research, Boost Care for LGBTQ Patients. Available online: https://www.heart.org/en/news/2020/10/08/heart-health-report-aims-to-bolster-research-boost-care-for-lgbtq-patients (accessed on 24 September 2023).
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Available online: https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/current-guidelines (accessed on 24 September 2023).
- American Heart Association. American Heart Association Recommendations for Physical Activity in Adults and Kids. Available online: https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-adults (accessed on 24 September 2023).
- Pahwa, S.; Deeks, S.; Zou, S.; Tomitch, N.M.; Miller-Novak, L.; Caler, E.; Justice, A.; Sacktor, N.; Gabuzda, D.; Hunt, P.W.; et al. NIH Workshop on HIV-Associated Comorbidities, Coinfections, and Complications: Summary and Recommendation for Future Research. Am. J. Ther. 2021, 86, 11–18. [Google Scholar] [CrossRef]
- Gabuzda, D.; Jamieson, B.D.; Collman, R.G.; Lederman, M.M.; Burdo, T.H.; Deeks, S.G.; Dittmer, D.P.; Fox, H.S.; Funderburg, N.T.; Pahwa, S.G.; et al. Pathogenesis of Aging and Age-related Comorbidities in People with HIV: Highlights from the HIV ACTION Workshop. Pathog. Immun. 2020, 5, 143–174. [Google Scholar] [CrossRef]
- Lagathu, C.; Cossarizza, A.; Béréziat, V.; Nasi, M.; Capeau, J.; Pinti, M. Basic science and pathogenesis of ageing with HIV: Potential mechanisms and biomarkers. AIDS 2017, 31 (Suppl. S2), S105–S119. [Google Scholar] [CrossRef] [PubMed]
- Walker, A. Nursing Ranked as the Most Trusted Profession for 22nd Year in a Row. Nursing.org. Available online: https://nurse.org/articles/nursing-ranked-most-honest-profession/ (accessed on 26 April 2024).
| Variables (N = 30) | Mean (SD) | Range |
|---|---|---|
| Age (year) | 47.47 (12.53) | 30–65 |
| N (%) | ||
| Race | Black | 3 (10) |
| Haitian a | 4 (13.3) | |
| Mixed or biracial | 13 (43.3) | |
| Unspecified b | 1 (3.3) | |
| Latinx c | 21 (70) | |
| US Born | 13 (43.3) | |
| Highest level of academic attainment | ||
| High school or GED d | 14 (46.7) | |
| Some college | 4 (13.3) | |
| 2- or 4-year degree | 6 (20) | |
| Graduate degree | 3 (10) | |
| Other | 3 (10) | |
| Employment | Part-time | 5 (16.7) |
| Full-time | 7 (23.3) | |
| Unemployed | 14 (46.7) | |
| Retired | 3 (10) | |
| Disabled | 1 (3.3) | |
| Annual income | ||
| Less than $20,000 | 14 (46.7) | |
| $20,001–$40,000 | 10 (33.3) | |
| $40,001–$60,000 | 6 (20) | |
| $60,001 and above | 0 (0) | |
| Relationship status | Partnered | 9 (30) |
| Single | 21 (70) | |
| Sleep Difficulties | % of Sleep Difficulties | Odds Ratio [95% CI] * | p-Value | |||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Stiff Joints | Yes | 6 | 5 | 54.5% | 2.06 [0.45, 9.30] | 0.3457 |
| No | 7 | 12 | 36.8% | |||
| Fatigue | Yes | 9 | 1 | 90.0% | 36.00 [3.47, 373.19] | 0.0003 |
| No | 4 | 16 | 20.0% | |||
| Upset Stomach | Yes | 7 | 2 | 77.8% | 8.75 [1.40, 54.80] | 0.0127 |
| No | 6 | 15 | 28.6% | |||
| Diarrhea | Yes | 6 | 3 | 66.7% | 4.00 [0.76, 20.96] | 0.0913 |
| No | 7 | 14 | 33.3% | |||
| Altered Sensation in Hands or Feet | Yes | 5 | 4 | 55.6% | 2.03 [0.42, 9.89] | 0.3765 |
| No | 8 | 13 | 38.1% | |||
| Pain | Yes | 5 | 3 | 62.5% | 2.92 [0.55, 15.56] | 0.2014 |
| No | 8 | 14 | 36.4% | |||
| Weight Loss | Yes | 5 | 3 | 62.5% | 2.92 [0.55, 15.56] | 0.2014 |
| No | 8 | 14 | 36.4% | |||
| Loss of Strength | Yes | 7 | 1 | 87.5% | 18.67 [1.88, 185.41] | 0.0094 |
| No | 6 | 16 | 27.3% | |||
| (N = 30) | Vigorous | Moderate | Walking | Sitting |
|---|---|---|---|---|
| Engagement | N (%) | N (%) | N (%) | N (%) |
| Days/week | ||||
| 0 | 15 (50) | 11 (36.7) | 0 (0) | |
| 1 | 1 (3.3) | 3 (10) | 1 (3.3) | N/A |
| 2 | 5 (16.7) | 5 (16.7) | 2 (6.7) | |
| 3 | 1 (3.3) | 4 (13.3) | 3 (10) | |
| 4 | 1 (3.3) | 1 (3.3) | 2 (6.7) | |
| 5 | 6 (20) | 2 (6.7) | 6 (20) | |
| 6 | 0 (0) | 0 (0) | 2 (6.7) | |
| 7 | 1 (3.3) | 4 (13.3) | 14 (46.7) | |
| Mean (SD) N = 15 | Mean (SD) N = 19 | Mean (SD) N = 30 | Mean (SD) N = 30 | |
| Days/week | 3.7 (1.8) | 3.5 (2.2) | 5.4 (1.9) | N/A |
| Minutes/day | 128 (134.6) | 94.2 (88.1) | 137.8 (178.5) | 384.8 (234.8) |
| Minutes/week | 512 (703) | 333.4 (398.3) | 883.3 (1274.6) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, S.R.; Kang, B.; Jeon, S.; Fraser, M.; Kershaw, T.; Boutjdir, M. Chronic Illness Perceptions and Cardiovascular Disease Risk Behaviors in Black and Latinx Sexual Minority Men with HIV: A Cross-Sectional Analysis. Nurs. Rep. 2024, 14, 1922-1936. https://doi.org/10.3390/nursrep14030143
Ramos SR, Kang B, Jeon S, Fraser M, Kershaw T, Boutjdir M. Chronic Illness Perceptions and Cardiovascular Disease Risk Behaviors in Black and Latinx Sexual Minority Men with HIV: A Cross-Sectional Analysis. Nursing Reports. 2024; 14(3):1922-1936. https://doi.org/10.3390/nursrep14030143
Chicago/Turabian StyleRamos, S. Raquel, Baram Kang, Sangchoon Jeon, Marilyn Fraser, Trace Kershaw, and Mohamed Boutjdir. 2024. "Chronic Illness Perceptions and Cardiovascular Disease Risk Behaviors in Black and Latinx Sexual Minority Men with HIV: A Cross-Sectional Analysis" Nursing Reports 14, no. 3: 1922-1936. https://doi.org/10.3390/nursrep14030143
APA StyleRamos, S. R., Kang, B., Jeon, S., Fraser, M., Kershaw, T., & Boutjdir, M. (2024). Chronic Illness Perceptions and Cardiovascular Disease Risk Behaviors in Black and Latinx Sexual Minority Men with HIV: A Cross-Sectional Analysis. Nursing Reports, 14(3), 1922-1936. https://doi.org/10.3390/nursrep14030143







