Interventions Effective in Decreasing Burden in Caregivers of Persons with Dementia: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy and Study Selection
2.2. Variables of Interest
2.3. Moderating Variables
2.4. Data Extraction
2.5. Data Analysis and Synthesis
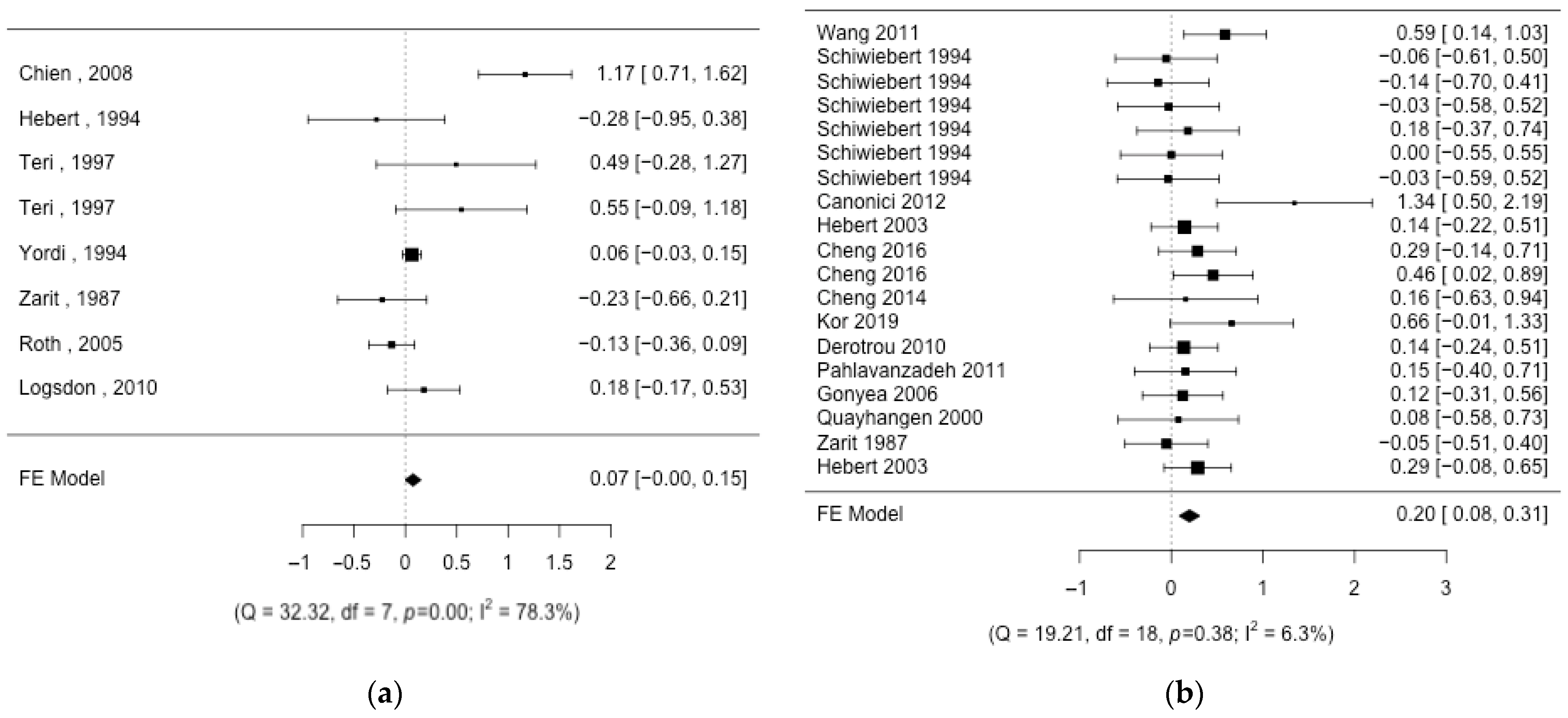
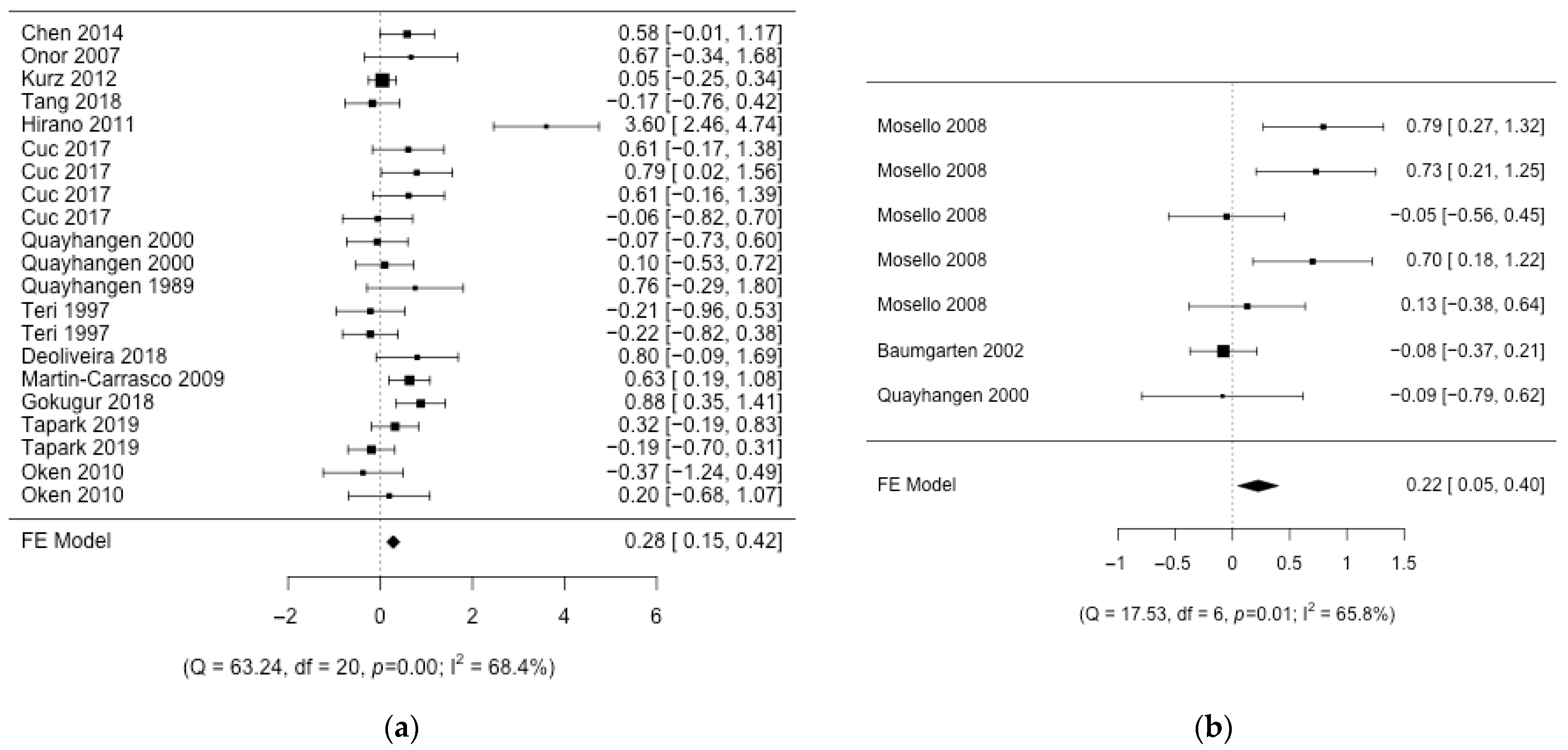
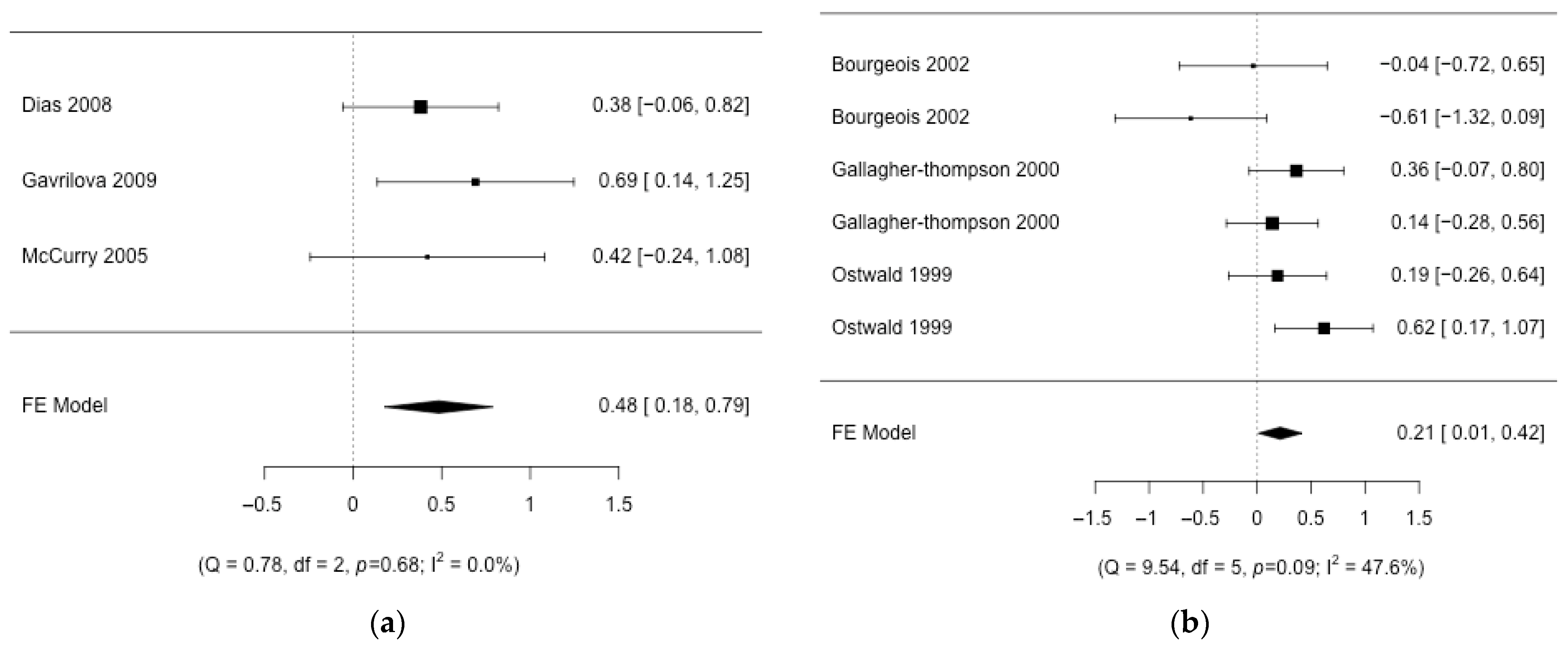
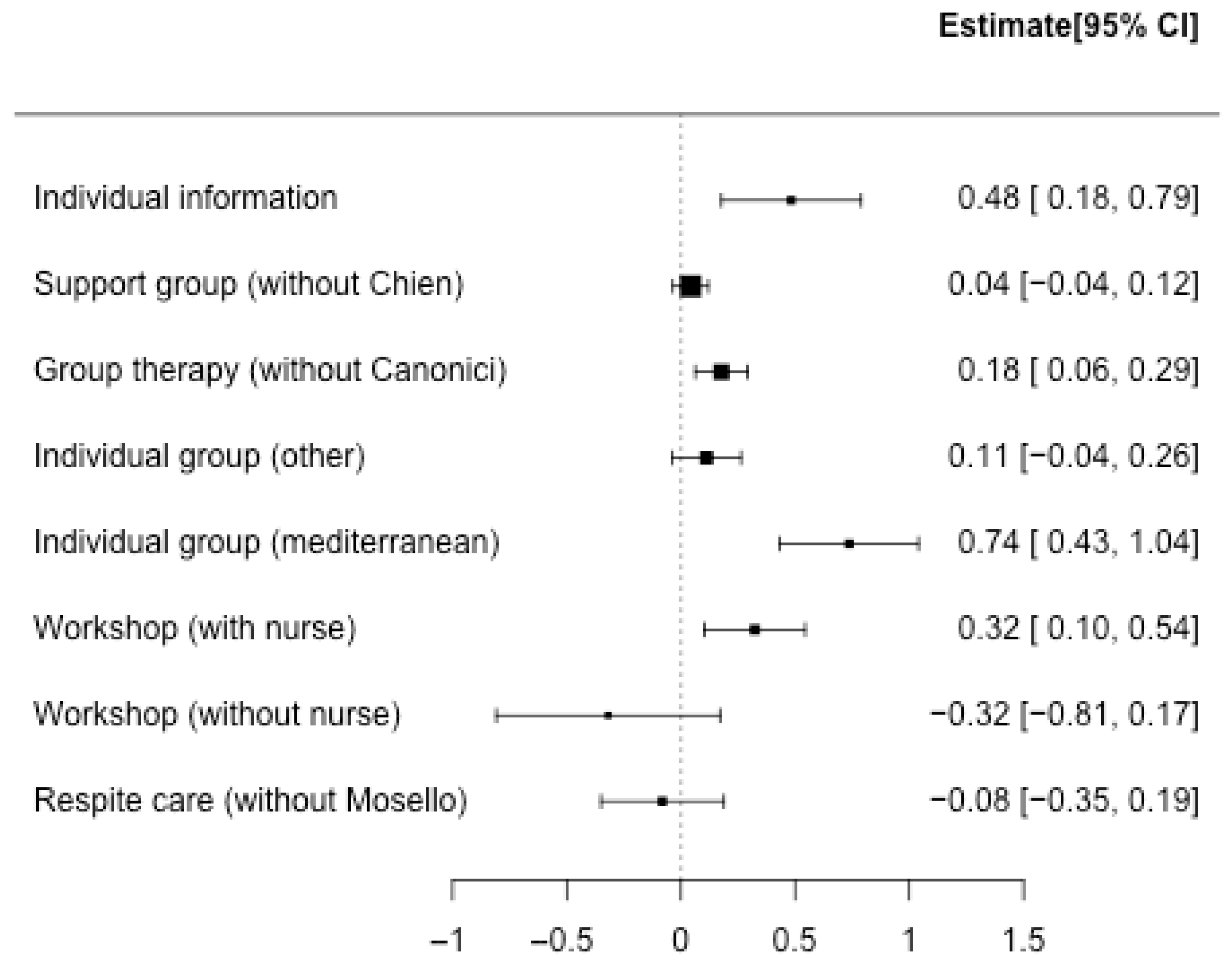
3. Results
3.1. Description of the Studies
3.2. Aggregation of Results
3.3. Risk of Bias
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Conflicts of Interest
References
- WHO. Enfermedades No Transmisibles. Available online: https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1 (accessed on 25 February 2019).
- WHO. OMS|10 Datos Sobre las Enfermedades No Transmisibles. Available online: https://www.who.int/es/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 24 February 2019).
- Dua, T.; Clark, N.; Al, E. Guía de Intervención mhGAP para los Trastornos Mentales, Neurológicos y por uso de Sustancias en el Nivel de Atención de la Salud no Especializada. Guía de Intervención mhGAP. Available online: https://www.who.int/es/publications/i/item/978924154806 (accessed on 2 February 2020).
- Centers for Disease Control and Prevention. About Dementia. National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Available online: https://www.cdc.gov/aging/alzheimers-disease-dementia/about-dementia.html#whatisdementia (accessed on 24 March 2024).
- Barbarino, P. Perspectiva Global Marzo de 2019. The Global Voice on Dementia. London March 2019, pp. 1–10. Available online: https://www.alzint.org/u/perspectiva-global-feb-2019.pdf (accessed on 24 March 2024).
- Organización Panamericana de la Salud. Demencia: Una Prioridad de Salud Publica; Washington, DC, USA, 2013. Available online: https://www.who.int/es (accessed on 24 March 2024).
- Simón, M.A.; Bueno, A.M.; Blanco, V.; Otero, P.; Vázquez, F.L. Sleep Disturbance, Psychological Distress and Perceived Burden in Female Family Caregivers of Dependent Patients with Dementia: A Case-Control Study. Healthcare 2022, 10, 2435. [Google Scholar] [CrossRef]
- Evans-lacko, S.; Bhatt, J.; Comas-herrera, A.A.; Amico, F.D.; Farina, N.; Gaber, S.; Knapp, P.M.; Salcher-konrad, M.; Stevens, M.; Wilson, E.; et al. Actitudes Hacia la Demencia Informe Mundial Sobre el Alzheimer 2019; London, UK, 2019. Available online: https://www.alzint.org/u/WorldAlzheimerReport2019-Spanish-Summary.pdf (accessed on 24 March 2024).
- Krishnamoorthy, E.S.; Gilliam, F. Best Clinical and Research Practice in Adult Epileptology. Epilepsy Behav. 2009, 15 (Suppl. S1), S55–S59. [Google Scholar] [CrossRef]
- Pinquart, M.; Sörensen, S. Differences between Caregivers and Noncaregivers in Psychological Health and Physical Health: A Meta-Analysis. Psychol. Aging 2003, 18, 250–267. [Google Scholar] [CrossRef]
- Brodaty, H.; Green, A.; Koschera, A. Meta-Analysis of Psychosocial Interventions for Caregivers of People with Dementia. J. Am. Geriatr. Soc. 2003, 51, 657–664. [Google Scholar] [CrossRef]
- Dunkin, J.J.; Anderson-Hanley, C. Dementia Caregiver Burden: A Review of the Literature and Guidelines for Assessment and Intervention. Neurology 1998, 51 (Suppl. S1), S53–S60. [Google Scholar] [CrossRef]
- Chien, L.-Y.; Chu, H.; Guo, J.-L.; Liao, Y.-M.; Chang, L.-I.; Chen, C.-H.; Chou, K.-R. Caregiver Support Groups in Patients with Dementia: A Meta-Analysis. Int. J. Geriatr. Psychiatry 2011, 26, 1089–1098. [Google Scholar] [CrossRef]
- Jensen, M.; Agbata, I.N.; Canavan, M.; McCarthy, G. Effectiveness of Educational Interventions for Informal Caregivers of Individuals with Dementia Residing in the Community: Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Geriatr. Psychiatry 2015, 30, 130–143. [Google Scholar] [CrossRef]
- Deeken, F.; Rezo, A.; Hinz, M.; Discher, R.; Rapp, M.A. Evaluation of Technology-Based Interventions for Informal Caregivers of Patients With Dementia—A Meta-Analysis of Randomized Controlled Trials. Am. J. Geriatr. Psychiatry 2019, 27, 426–445. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Lira, R.P.C.; Rocha, E.M. PICOT: Imprescriptible Items in a Clinical Research Question. Arq. Bras. Oftalmol. 2019, 82, V. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Novak, M.; Guest, C. Caregiver Response to Alzheimer’s Disease. Int. J. Aging Hum. Dev. 1989, 28, 67–79. [Google Scholar] [CrossRef]
- Bourgeois, M.; Schulz, R.; Burgio, L.; Beach, S. Skills Training for Spouses of Patients with Alzheimer’s Disease: Outcomes of an Intervention Study. J. Clin. Geropsychology 2002, 8, 53–73. [Google Scholar] [CrossRef]
- Poulshock, S.W.; Deimling, G.T. Families Caring for Elders in Residence: Issues in the Measurement of Burden. J. Gerontol. 1984, 39, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Zarit, S.H.; Orr, N.; Zarit, J.M. Families under Stress: Caring for the Patient with Alzheimer’s Disease and Related Disorders; New York University Press: New York, NY, USA, 1985. [Google Scholar]
- Zarit, S.H.; Reever, K.E.; Bach-Peterson, J. Relatives of the Impaired Elderly: Correlates of Feelings of Burden. Gerontologist 1980, 20, 649–655. [Google Scholar] [CrossRef]
- Teri, L.; Truax, P.; Logsdon, R.; Uomoto, J.; Zarit, S.; Vitaliano, P.P. Assessment of Behavioral Problems in Dementia: The Revised Memory and Behavior Problems Checklist. Psychol. Aging 1992, 7, 622–631. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Verhagen, A.P.; De Vet, H.C.W.; De Bie, R.A.; Kessels, A.G.H.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Peacock, S.C.; Forbes, D.A. Interventions for Caregivers of Persons with Dementia: A Systematic Review. Can. J. Nurs. Res. 2003, 35, 88–107. [Google Scholar]
- McCurry, S.M.; Gibbons, L.E.; Logsdon, R.G.; Vitiello, M.V.; Teri, L. Nighttime Insomnia Treatment and Education for Alzheimer’s Disease: A Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2005, 53, 793–802. [Google Scholar] [CrossRef]
- Dias, A.; Dewey, M.E.; D’Souza, J.; Dhume, R.; Motghare, D.D.; Shaji, K.S.; Menon, R.; Prince, M.; Patel, V. The Effectiveness of a Home Care Program for Supporting Caregivers of Persons with Dementia in Developing Countries: A Randomised Controlled Trial from Goa, India. PLoS ONE 2008, 3, e2333. [Google Scholar] [CrossRef]
- Gavrilova, S.; Ferri, C.; Mikhaylova, N.; Sokolova, O.; Al, E. Helping Carers to Care—The 10/66 Dementia Research Group’s Randomized Control Tiral of a Caregiver Intervention in Russia. Int. J. Geriatr. Psychiatry 2009, 24, 347–354. [Google Scholar] [CrossRef]
- Quayhagen, M.P.; Quayhagen, M. Differential Effects of Family-Based Strategies on Alzheimer’s Disease. Gerontologist 1989, 29, 150–155. [Google Scholar] [CrossRef]
- Teri, L.; Logsdon, R.; Uomoto, J.; Mc Curry, S. Behavioral Treatment of Depression in Dementia Patients: A Controlled Clinical Trial. J. Am. Geriatr. Soc. 1997, 45, 1542–1543. [Google Scholar] [CrossRef]
- Quayhagen, M.P.; Quayhagen, M.; Corbeil, R.R.; Hendrix, R.C.; Jackson, J.E.; Snyder, L.; Bower, D. Coping with Dementia: Evaluation of Four Nonpharmacologic Interventions. Int. Psychogeriatr. 2000, 12, 249–265. [Google Scholar] [CrossRef]
- Onor, M.L.; Trevisiol, M.; Negro, C.; Alessandra, S.; Saina, M.; Aguglia, E. Impact of a Multimodal Rehabilitative Intervention on Demented Patients and Their Caregivers. Am. J. Alzheimer’s Dis. Other Dementiasr. 2007, 22, 261–272. [Google Scholar] [CrossRef]
- Martin-Carrasco, M.; Franco Martin, M.; Pelegrin Valero, C.; Roy Millan, P.; Al, E. Improvement in Behavioral Symptoms in Patients with Moderate to Servere Alzheimer’s. Int. J. Geriatr. Psychiatry 2009, 24, 489–499. [Google Scholar] [CrossRef]
- Oken, B.S.; Fonareva, I.; Haas, M.; Wahbeh, H.; Lane, J.B.; Zajdel, D.; Amen, A. Pilot Controlled Trial of Mindfulness Meditation and Education for Dementia Caregivers. J. Altern. Complement. Med. 2010, 16, 1031–1038. [Google Scholar] [CrossRef]
- Hirano, A.; Suzuki, Y.; Kuzuya, M.; Onishi, J.; Ban, N.; Umegaki, H. Influence of Regular Exercise on Subjective Sense of Burden and Physical Symptoms in Community-Dwelling Caregivers of Dementia Patients: A Randomized Controlled Trial. Arch. Gerontol. Geriatr. 2011, 53, e158–e163. [Google Scholar] [CrossRef]
- Kurz, A.; Thöne-Otto, A.; Cramer, B.; Egert, S.; Frölich, L.; Gertz, H.J.; Kehl, V.; Wagenpfeil, S.; Werheid, K. CORDIAL: Cognitive Rehabilitation and Cognitive-Behavioral Treatment for Early Dementia in Alzheimer Disease: A Multicenter, Randomized, Controlled Trial. Alzheimer Dis. Assoc. Disord. 2012, 26, 246–253. [Google Scholar] [CrossRef]
- Chen, H.M.; Huang, M.F.; Yeh, Y.C.; Huang, W.H.; Chen, C.S. Effectiveness of Coping Strategies Intervention on Caregiver Burden among Caregivers of Elderly Patients with Dementia. Psychogeriatrics 2015, 15, 20–25. [Google Scholar] [CrossRef]
- Cuc, A.V.; Locke, D.E.C.; Duncan, N.; Fields, J.A.; Snyder, C.H.; Hanna, S.; Lunde, A.; Smith, G.E.; Chandler, M. A Pilot Randomized Trial of Two Cognitive Rehabilitation Interventions for Mild Cognitive Impairment: Caregiver Outcomes. Int. J. Geriatr. Psychiatry 2017, 32, e180–e187. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; Radanovic, M.; Homem de Mello, P.C.; Buchain, P.C.; Dias Vizzotto, A.; Harder, J.; Stella, F.; Piersol, C.V.; Gitlin, L.N.; Forlenza, O.V. An Intervention to Reduce Neuropsychiatric Symptoms and Caregiver Burden in Dementia: Preliminary Results from a Randomized Trial of the Tailored Activity Program–Outpatient Version. Int. J. Geriatr. Psychiatry 2018, 34, 1301–1307. [Google Scholar] [CrossRef]
- Gok Ugur, H.; Orak, O.S.; Yaman Aktas, Y.; Enginyurt, O.; Saglambilen, O. Effects of Music Therapy on the Care Burden of In-Home Caregivers and Physiological Parameters of Their in-Home Dementia Patients: A Randomized Controlled Trial. Complement. Med. Res. 2018, 26, 22–30. [Google Scholar] [CrossRef]
- Tang, S.H.; Chio, O.I.; Chang, L.H.; Mao, H.F.; Chen, L.H.; Yip, P.K.; Hwang, J.P. Caregiver Active Participation in Psychoeducational Intervention Improved Caregiving Skills and Competency. Geriatr. Gerontol. Int. 2018, 18, 750–757. [Google Scholar] [CrossRef]
- Ta Park, V.M.; Ton, V.; Tiet, Q.Q.; Vuong, Q.; Yeo, G.; Gallagher-Thompson, D. Promising Results from a Pilot Study to Reduce Distress in Vietnamese American Dementia and Memory Loss Caregivers. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 319–327. [Google Scholar] [CrossRef]
- Zarit, S.H.; Anthony, C.R.; Boutselis, M. Interventions with Care Givers of Dementia Patients: Comparison of Two Approaches. Psychol. Aging 1987, 2, 225–232. [Google Scholar] [CrossRef]
- Schwiebert, V.L.; Myers, J.E. Midlife Care Givers: Effectiveness of a Psychoeducational Intervention for Midlife Adults with Parent-Care Responsibilities. J. Couns. Dev. 1994, 72, 627–632. [Google Scholar] [CrossRef]
- Hébert, R.; Lévesque, L.; Vézina, J.; Lavoie, J.P.; Ducharme, F.; Gendron, C.; Préville, M.; Voyer, L.; Dubois, M.F. Efficacy of a Psychoeducative Group Program for Caregivers of Demented Persons Living at Home: A Randomized Controlled Trial. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2003, 58, 58–67. [Google Scholar] [CrossRef]
- Gonyea, J.G.; O’Connor, M.K.; Boyle, P.A. Project CARE: A Randomized Controlled Trial of a Behavioral Intervention Group for Alzheimer’s Disease Caregivers. Gerontologist 2006, 46, 827–832. [Google Scholar] [CrossRef]
- De Rotrou, J.; Cantegreil, I.; Faucounau, V.; Wenisch, E.; Chausson, C.; Jegou, D.; Grabar, S.; Rigaud, A.S. Do Patients Diagnosed with Alzheimer’s Disease Benefit from a Psycho-Educational Programme for Family Caregivers? A Randomised Controlled Study. Int. J. Geriatr. Psychiatry 2011, 26, 833–842. [Google Scholar] [CrossRef]
- Pahlavanzadeh, S.; Heidari, F.G.; Maghsudi, J.; Ghazavi, Z.; Samandari, S. The Effects of Family Education Program on the Caregiver Burden of Families of Elderly with Dementia Disorders. Iran. J. Nurs. Midwifery Res. 2010, 15, 102–108. [Google Scholar]
- Wang, L.Q.; Chien, W.T. Randomised Controlled Trial of a Family-Led Mutual Support Programme for People with Dementia. J. Clin. Nurs. 2011, 20, 2362–2366. [Google Scholar] [CrossRef]
- Canonici, A.P.; de Andrade, L.P.; Gobbi, S.; Santos-Galduroz, R.F.; Gobbi, L.T.B.; Stella, F. Functional Dependence and Caregiver Burden in Alzheimer’s Disease: A Controlled Trial on the Benefits of Motor Intervention. Psychogeriatrics 2012, 12, 186–192. [Google Scholar] [CrossRef]
- Cheng, S.T.; Fung, H.H.; Chan, W.C.; Lam, L.C.W. Short-Term Effects of a Gain-Focused Reappraisal Intervention for Dementia Caregivers: A Double-Blind Cluster-Randomized Controlled Trial. Am. J. Geriatr. Psychiatry 2016, 24, 740–750. [Google Scholar] [CrossRef]
- Cheng, S.-T.; Lau, R.W.L.; Mak, E.P.M.; Ng, N.S.S.; Lam, L.C.W. Benefit-Finding Intervention for Alzheimer Caregivers: Conceptual Framework, Implementation Issues, and Preliminary Efficacy. Gerontologist 2014, 54, 1049–1058. [Google Scholar] [CrossRef]
- Kor, P.P.K.; Liu, J.Y.W.; Chien, W.T. Effects of a Modified Mindfulness-Based Cognitive Therapy for Family Caregivers of People with Dementia: A Pilot Randomized Controlled Trial; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 98. [Google Scholar] [CrossRef]
- Baumgarten, M.; Lebel, P.; Laprise, H.; Leclerc, C.; Quinn, C. Adult Day Care for the Frail Elderly. J. Aging Health 2002, 14, 237–259. [Google Scholar] [CrossRef]
- Mossello, E.; Caleri, V.; Razzi, E.; Di Bari, M.; Cantini, C.; Tonon, E.; Lopilato, E.; Marini, M.; Simoni, D.; Cavallini, M.C.; et al. Day Care for Older Dementia Patients: Favorable Effects on Behavioral and Psychological Symptoms and Caregiver Stress. Int. J. Geriatr. Psychiatry 2008, 23, 1066–1072. [Google Scholar] [CrossRef]
- Hébert, R.; Leclerc, G.; Bravo, G.; Girouard, D.; Lefrançois, R. Efficacy of a Support Group Programme for Care-Givers of Demented Patients in the Community: A Randomized Controlled Trial. Arch. Gerontol. Geriatr. 1994, 18, 1–14. [Google Scholar] [CrossRef]
- Yordi, C.; Newcomer, R.; DuNah, R.; Fox, P.; Wilkinson, A. Effects of the Medicare Alzheimer’s Disease Demonstration on Caregiver Burden and Depression. Health Serv. Res. 1999, 34, 669–689. [Google Scholar]
- Roth, D.L.; Mittelman, M.S.; Clay, O.J.; Madan, A.; Haley, W.E. Changes in Social Support as Mediators of the Impact of a Psychosocial Intervention for Spouse Caregivers of Persons with Alzheimer’s Disease. Psychol. Aging 2005, 20, 634–644. [Google Scholar] [CrossRef]
- Chien, W.T.; Lee, Y.M. A Disease Management Program for Families of Persons in Hong Kong with Dementia. Psychiatr. Serv. 2008, 59, 433–436. [Google Scholar] [CrossRef]
- Logsdon, R.G.; Pike, K.C.; McCurry, S.M.; Hunter, P.; Maher, J.; Snyder, L.; Teri, L. Early-Stage Memory Loss Support Groups: Outcomes from a Randomized Controlled Clinical Trial. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2010, 65, 691–697. [Google Scholar] [CrossRef]
- Ostwald, S.K.; Hepburn, K.W.; Caron, W.; Burns, T.; Mantell, R. Reducing Caregiver Burden: A Randomized Psychoeducational Intervention for Caregivers of Persons with Dementia. Gerontologist 1999, 39, 299–309. [Google Scholar] [CrossRef]
- Gallagher-Thompson, D.; Lovett, S.; Rose, J.; McKibbin, C.; Coon, D.; Futterman, A.; Thompson, L. Impact of Psychoeducational Interventions on Distressed Family Caregivers. J. Clin. Geropsychology 2000, 6, 91–110. [Google Scholar] [CrossRef]
- Schulz, R. Some Critical Issues in Caregiver Intervention Research. Aging Ment. Health 2001, 5 (Suppl. S1), 112–115. [Google Scholar] [CrossRef]
- Ortega, Z.; Martín-Vallejo, J.; Mencía, A.; Galindo-Villardón, M.P.; Pérez-Mellado, V. Introducing Meta-Partition, a Useful Methodology to Explore Factors That Influence Ecological Effect Sizes. PLoS ONE 2016, 11, e0158624. [Google Scholar] [CrossRef]
- Hedges, L.V. Statistical Methodology in Meta-Analysis; Academic Press: Chicago, IL, USA, 1982. Available online: https://files.eric.ed.gov/fulltext/ED227133.pdf (accessed on 24 March 2024).
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Routledge: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Langhorne, P. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Prospectively Identified Trials Could Be Used for Comparison with Meta-Analyses. BMJ 1998, 316, 471. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, 4898. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Del Re, A.C.; Hoyt, W. MAd: Meta-Analysis with Mean Differences. R Package. Available online: https://cran.r-project.org/package=MAd (accessed on 24 February 2019).
- Del Re, A. RcmdrPlugin.MA: Graphical User Interface for Conduting Meta-Analysis in R. R Package. Available online: http://cran.r-project.org/web/packages/RcmdrPlugin.MA (accessed on 24 February 2019).
- JMP, version 16; SAS Institute Inc.: Cary, NC, USA, 1989–2019.
- B.O.E. Ley 44/2003, de 21 de Noviembre, de Ordenación de Las Profesiones; Spain, 2003; p. 29. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2003-21340 (accessed on 24 March 2024).
- Abrahams, R.; Liu, K.P.Y.; Bissett, M.; Fahey, P.; Cheung, K.S.L.; Bye, R.; Chaudhary, K.; Chu, L.-W. Effectiveness of Interventions for Co-Residing Family Caregivers of People with Dementia: Systematic Review and Meta-Analysis. Aust. Occup. Ther. J. 2018, 65, 208–224. [Google Scholar] [CrossRef]
- Pinquart, M.; Sörensen, S. Helping Caregivers of Persons with Dementia: Which Interventions Work and How Large Are Their Effects? Int. Psychogeriatr. 2006, 18, 577–595. [Google Scholar] [CrossRef]
- Knight, B.G.; Lutzky, S.M.; Macofsky-Urban, F. A Meta-Analytic Review of Interventions for Caregiver Distress: Recommendations for Future Research. Gerontologist 1993, 33, 240–248. [Google Scholar] [CrossRef]
- Schulz, R.; O’ Brien, A.; Czaja, S.; Ory, M.; Norris, R.; Martire, L.M.; Belle, S.H.; Burgio, L.; Gitlin, L.; Coon, D.; et al. Dementia Caregiver Intervention Research: In Search of Clinical Significance. Gerontologist 2002, 42, 589–602. [Google Scholar] [CrossRef]
- Sorensen, S.; Pinquart, M.; Duberstein, P. How Effective Are Interventions with Caregivers? An Updated Meta-Analysis. Gerontologist 2002, 42, 356–372. [Google Scholar] [CrossRef]
- Gibson, G.D. Racial, Ethnic, and Cultural Differences in Dementia Caregiving: Review and Analysis. Gerontologist 1997, 37, 355–364. [Google Scholar] [CrossRef]
- Powell Lawton, M.; Brody, E.M.; Saperstein, A.R. A Controlled Study of Respite Service for Caregivers of Alzheimer’s Patients. Gerontologist 1989, 29, 8–16. [Google Scholar] [CrossRef]
- Hooyman, N.; Gonyea, J.; Montgomery, R. The Impact of In-Home Services Termination on Family Caregivers. Gerontologist 1985, 25, 141–145. [Google Scholar] [CrossRef]
- Lovett, S.; Gallagher, D. Psychoeducational Interventions for Family Caregivers: Preliminary Efficacy Data. Behav. Ther. 1988, 19, 321–330. [Google Scholar] [CrossRef]
- Toseland, R.W.; Rossiter, C.M. Group Interventions to Support Family Caregivers: A Review and Analysis. Gerontologist 1989, 29, 438–448. [Google Scholar] [CrossRef]
- Annual reports: Family Survival Program Pilot Project. In Family Survival Project (1981–1984); Family Survival Project: San Francisco, CA, USA, 1984.
- Flanagan, S.; Damery, S.; Combes, G. The Effectiveness of Integrated Care Interventions in Improving Patient Quality of Life (QoL) for Patients with Chronic Conditions. An Overview of the Systematic Review Evidence. Health Qual. Life Outcomes 2017, 15, 188. [Google Scholar] [CrossRef]
- Boots, L.M.M.; de Vugt, M.E.; van Knippenberg, R.J.M.; Kempen, G.I.J.M.; Verhey, F.R.J. A Systematic Review of Internet-Based Supportive Interventions for Caregivers of Patients with Dementia. Int. J. Geriatr. Psychiatry 2014, 29, 331–344. [Google Scholar] [CrossRef]
- Feeny, D.; Furlong, W.; Boyle, M.; Torrance, G.W. Multi-Attribute Health Status Classification Systems. Pharmacoeconomics 1995, 7, 490–502. [Google Scholar] [CrossRef]
- Jones, C.; Edwards, R.T.; Hounsome, B. Health Economics Research into Supporting Carers of People with Dementia: A Systematic Review of Outcome Measures. Health Qual. Life Outcomes 2012, 10, 142. [Google Scholar] [CrossRef]
- Neumann, P.J.; Kuntz, K.M.; Leon, J.; Araki, S.S.; Hermann, R.C.; Hsu, M.-A.; Weinstein, M.C. Health Utilities in Alzheimer’s Disease. Med. Care 1999, 37, 27–32. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Alcázar, F.J.; Juárez-Vela, R.; Sánchez-González, J.L.; Martín-Vallejo, J. Interventions Effective in Decreasing Burden in Caregivers of Persons with Dementia: A Meta-Analysis. Nurs. Rep. 2024, 14, 931-945. https://doi.org/10.3390/nursrep14020071
Rodríguez-Alcázar FJ, Juárez-Vela R, Sánchez-González JL, Martín-Vallejo J. Interventions Effective in Decreasing Burden in Caregivers of Persons with Dementia: A Meta-Analysis. Nursing Reports. 2024; 14(2):931-945. https://doi.org/10.3390/nursrep14020071
Chicago/Turabian StyleRodríguez-Alcázar, Francisco José, Raúl Juárez-Vela, Juan Luis Sánchez-González, and Javier Martín-Vallejo. 2024. "Interventions Effective in Decreasing Burden in Caregivers of Persons with Dementia: A Meta-Analysis" Nursing Reports 14, no. 2: 931-945. https://doi.org/10.3390/nursrep14020071
APA StyleRodríguez-Alcázar, F. J., Juárez-Vela, R., Sánchez-González, J. L., & Martín-Vallejo, J. (2024). Interventions Effective in Decreasing Burden in Caregivers of Persons with Dementia: A Meta-Analysis. Nursing Reports, 14(2), 931-945. https://doi.org/10.3390/nursrep14020071






