Non-Pharmacological Management of Urge Urinary Incontinence in Women between 40 and 65 Years Old: A Systematic Review
Abstract
1. Introduction
Objectives
- (I).
- What types of non-pharmacological practices have been employed to reduce or prevent UUI in women between 40 and 65 years old?
- (II).
- What is the effectiveness of these interventions?
2. Materials and Methods
2.1. Study Protocol
2.2. Criteria for Considering Studies for This Review
2.2.1. Types of Participants
2.2.2. Types of Interventions
2.2.3. Types of Outcome Measures
2.2.4. Types of Studies
2.3. Search Strategies
2.4. Study Selection
2.5. Assessment of Methodological Quality
2.6. Data Extraction
2.7. Data Synthesis
3. Results
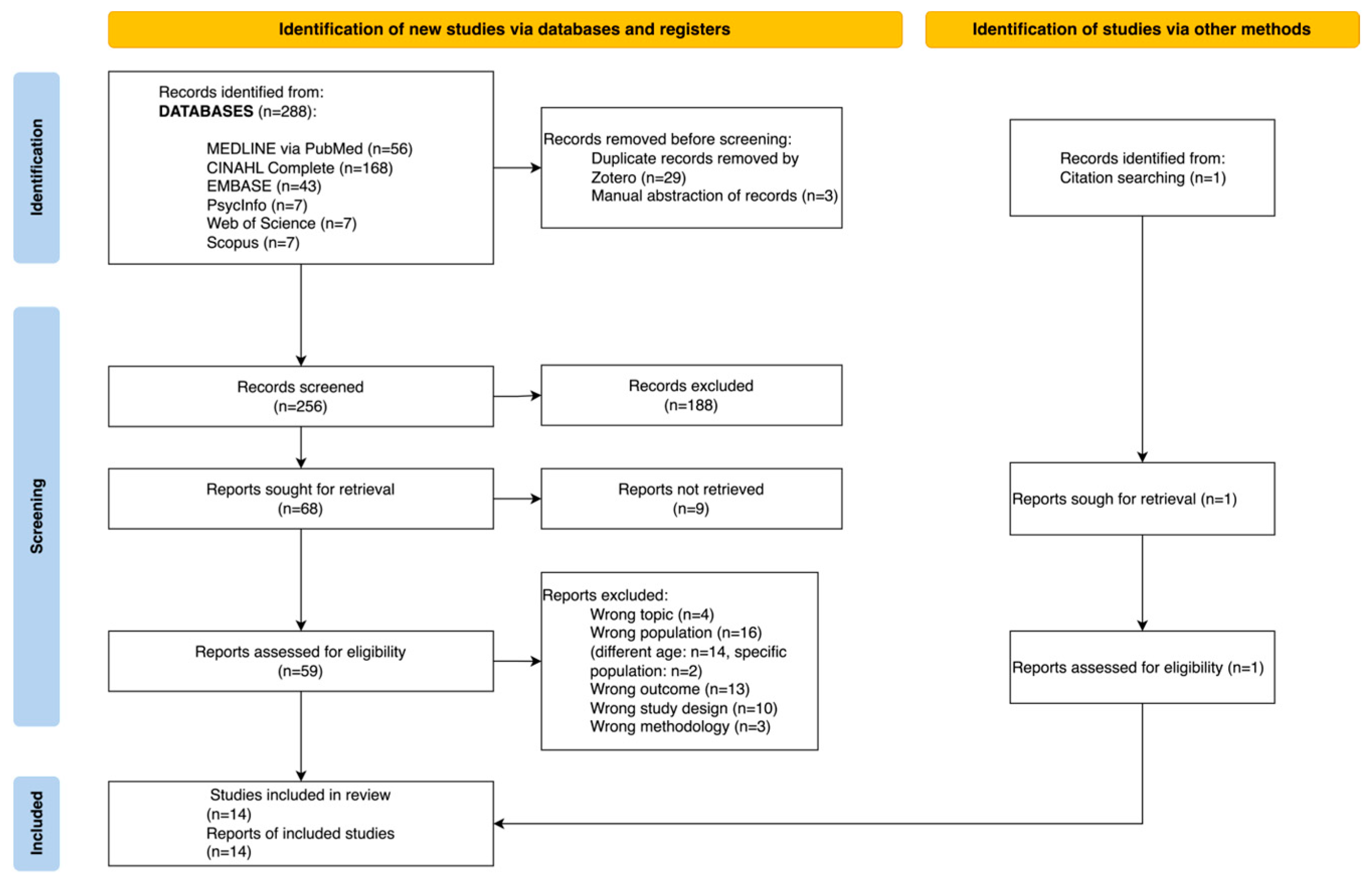
3.1. Description of the Studies
3.2. Methodological Quality
3.3. Physical Interventions
3.3.1. Pelvic Floor Muscle Training and Physiotherapy
3.3.2. Bladder Training
3.4. Physical and Psycho-Educational Interventions
Behavioral Training
3.5. Psychological Interventions
Hypnotherapy
3.6. Healthcare Professionals Involved and Setting
3.7. Other Outcomes
3.8. Adverse Events
4. Discussion
Limitations
5. Conclusions
Implications for Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Conflicts of Interest
References
- Todhunter-Brown, A.; Hazelton, C.; Campbell, P.; Elders, A.; Hagen, S.; McClurg, D. Conservative Interventions for Treating Urinary Incontinence in Women: An Overview of Cochrane Systematic Reviews. Cochrane Database Syst. Rev. 2022, 9, CD012337. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The Standardisation of Terminology in Lower Urinary Tract Function: Report from the Standardisation Sub-Committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- Haylen, B.T.; De Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Bo, K.; Frawley, H.C.; Haylen, B.T.; Abramov, Y.; Almeida, F.G.; Berghmans, B.; Bortolini, M.; Dumoulin, C.; Gomes, M.; McClurg, D.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for the Conservative and Nonpharmacological Management of Female Pelvic Floor Dysfunction. Int. Urogynecol. J. 2017, 28, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Parpio, Y.N.; Minaz, A.; Haider, S.I. Urinary Incontinence: Understanding the Silent Plight of Women. J. Coll. Physicians Surg. Pak. JCPSP 2022, 32, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Kopp, Z.S.; Agatep, B.; Milsom, I.; Abrams, P. Worldwide Prevalence Estimates of Lower Urinary Tract Symptoms, Overactive Bladder, Urinary Incontinence and Bladder Outlet Obstruction: WORLDWIDE PREVALENCE OF LUTS. BJU Int. 2011, 108, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.N.; Puckett, Y. Urinary Incontinence. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Aslan, E.; Beji, N.K.; Erkan, H.A.; Yalcin, O.; Gungor, F. Urinary Incontinence (UI) and Quality of Life (QoL) of the Elderly Residing in Residential Homes in Turkey. Arch. Gerontol. Geriatr. 2009, 49, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Felsted, K.F.; Supiano, K.P. Mindfulness-Based Stress Reduction Versus a Health Enhancement Program in the Treatment of Urge Urinary Incontinence in Older Adult Women: A Randomized Controlled Feasibility Study. Res. Gerontol. Nurs. 2019, 12, 285–297. [Google Scholar] [CrossRef]
- Riss, P.; Kargl, J. Quality of Life and Urinary Incontinence in Women. Maturitas 2011, 68, 137–142. [Google Scholar] [CrossRef]
- Milsom, I.; Coyne, K.S.; Nicholson, S.; Kvasz, M.; Chen, C.-I.; Wein, A.J. Global Prevalence and Economic Burden of Urgency Urinary Incontinence: A Systematic Review. Eur. Urol. 2014, 65, 79–95. [Google Scholar] [CrossRef]
- Siracusano, S.; Pregazzi, R.; d’Aloia, G.; Sartore, A.; Di Benedetto, P.; Pecorari, V.; Guaschino, S.; Pappagallo, G.; Belgrano, E. Prevalence of Urinary Incontinence in Young and Middle-Aged Women in an Italian Urban Area. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 107, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.S.; Wein, A.; Nicholson, S.; Kvasz, M.; Chen, C.-I.; Milsom, I. Economic Burden of Urgency Urinary Incontinence in the United States: A Systematic Review. J. Manag. Care Pharm. 2014, 20, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W.; et al. Population-Based Survey of Urinary Incontinence, Overactive Bladder, and Other Lower Urinary Tract Symptoms in Five Countries: Results of the EPIC Study. Eur. Urol. 2006, 50, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Wieland, L.S.; Shrestha, N.; Lassi, Z.S.; Panda, S.; Chiaramonte, D.; Skoetz, N. Yoga for Treating Urinary Incontinence in Women. Cochrane Database Syst. Rev. 2019, 2019, CD012668. [Google Scholar] [CrossRef]
- Fincopp—Federazione Italiana Incontinenti e Disfunzioni Pavimento Pelvico. Available online: https://fincopp.org/ (accessed on 14 October 2023).
- Murukesu, R.R.; Singh, D.K.A.; Shahar, S. Urinary Incontinence among Urban and Rural Community Dwelling Older Women: Prevalence, Risk Factors and Quality of Life. BMC Public Health 2019, 19, 529. [Google Scholar] [CrossRef]
- Ganz, M.L.; Smalarz, A.M.; Krupski, T.L.; Anger, J.T.; Hu, J.C.; Wittrup-Jensen, K.U.; Pashos, C.L. Economic Costs of Overactive Bladder in the United States. Urology 2010, 75, 526–532.e18. [Google Scholar] [CrossRef] [PubMed]
- Press Release: Incontinence Costs European Society over 40 Billion Euros per Year. Available online: https://mailchi.mp/uroweb/urology22-week-you-can-make-a-difference-301153?e=502f876984 (accessed on 19 October 2023).
- Wagner, T.H.; Hu, T.-W. Economic Costs of Urinary Incontinence in 1995. J. Urol. 1998, 51, 355–361. [Google Scholar] [CrossRef]
- Palese, A.; Carniel, G. The Effects of a Multi-Intervention Incontinence Care Program on Clinical, Economic, and Environmental Outcomes. J. Wound. Ostomy Cont. Nurs. 2011, 38, 177–183. [Google Scholar] [CrossRef]
- Tiwari, A.; Naruganahalli, K.S. Current and Emerging Investigational Medical Therapies for the Treatment of Overactive Bladder. Expert Opin. Investig. Drugs 2006, 15, 1017–1037. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis; Joanna Briggs Institute: Adelaide, Australia, 2020; ISBN 978-0-648-84880-6. [Google Scholar]
- Counsell, C. Formulating Questions and Locating Primary Studies for Inclusion in Systematic Reviews. Ann. Intern. Med. 1997, 127, 380–387. [Google Scholar] [CrossRef]
- Woodley, S.J.; Lawrenson, P.; Boyle, R.; Cody, J.D.; Mørkved, S.; Kernohan, A.; Hay-Smith, E.J.C. Pelvic Floor Muscle Training for Preventing and Treating Urinary and Faecal Incontinence in Antenatal and Postnatal Women. Cochrane Database Syst. Rev. 2020, 2021, CD007471. [Google Scholar] [CrossRef]
- Mueen Ahmed, K.K.; Dhubaib, B.E.A. Zotero: A Bibliographic Assistant to Researcher. J. Pharmacol. Pharmacother. 2011, 2, 304–305. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The Revised JBI Critical Appraisal Tool for the Assessment of Risk of Bias for Randomized Controlled Trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic Reviews of Effectiveness. In JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020; ISBN 978-0-648-84880-6. [Google Scholar]
- Chaboyer, W.; Harbeck, E.; Lee, B.-O.; Grealish, L. Missed Nursing Care: An Overview of Reviews. Kaohsiung J. Med. Sci. 2021, 37, 82–91. [Google Scholar] [CrossRef]
- Anonymous. Pelvic Floor Muscle Exercises Effective for Urinary Incontinence in Women. Am. Nurse. 2012. Available online: https://www.myamericannurse.com/pelvic-floor-muscle-exercises-effective-for-urinary-incontinence-in-women/ (accessed on 14 October 2023).
- Baker, J. Mindfulness-Based Stress Reduction Techniques and Yoga for Treatment of Urinary Urge Incontinence (MBSR-Yoga). Female Pelvic Med. Reconstr. Surg. 2014, 18, 46–49. [Google Scholar] [CrossRef]
- Elder, J.S. Bladder Rehabilitation, the Effect of a Cognitive Training Programme on Urge Incontinence. J. Urol. 1997, 158, 1642. [Google Scholar]
- Fay, M.F. A Practical Approach to Managing Incontinence. Todays OR Nurse 1988, 10, 8–38. [Google Scholar]
- Felsted, K. Comparing Mindfulness-Based Stress Reduction with the Health Enhancement Program in the Treatment of Urinary Urge Incontinence in Older Adult Women: A Pilot Feasibility and Randomized Controlled Trial. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, 2019. [Google Scholar]
- Gerard, L. Group Learning Behavior Modification and Exercise for Women with Urinary Incontinence. Urol. Nurs. 1997, 17, 17–22. [Google Scholar]
- Marlow, L. Bathroom Blues? Urinary Incontinence Can Be Managed. CURE Cancer Updat. Res. Educ. 2012, 11, 24. [Google Scholar]
- Yeung, Y.M.; Leung, F.N.Y.; Lam, M.C.; Yuen, A.C.M. A Nurse-Led Ward-Based Continence Rehabilitation Program in Geriatric Hospital. Hong Kong Nurs. J. 2002, 38, 22–28. [Google Scholar]
- Wang, A.C. Bladder-Sphincter Biofeedback as Treatment of Detrusor Instability in Women Who Failed to Respond to Oxybutynin. Chang. Gung Med. J. 2000, 23, 590–599. [Google Scholar] [PubMed]
- Wadensten, T.; Nyström, E.; Franzén, K.; Lindam, A.; Wasteson, E.; Samuelsson, E. A Mobile App for Self-Management of Urgency and Mixed Urinary Incontinence in Women: Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e19439. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Kafri, R.; Langer, R.; Dvir, Z.; Katz-Leurer, M. Rehabilitation vs Drug Therapy for Urge Urinary Incontinence: Short-Term Outcome. Int. Urogynecol. J. 2007, 18, 407–411. [Google Scholar] [CrossRef]
- Lagro-Janssen, T.; van Weel, C. Long-Term Effect of Treatment of Female Incontinence in General Practice. Br. J. Gen. Pract. 1998, 48, 1735–1738. [Google Scholar] [PubMed]
- Singh, N.; Arya, K.N. Effectiveness of Bladder Rehabilitation Program in the Management of Urge Urinary Incontinence in Older Women. Indian J. Physiother. Occup. Ther. 2011, 5, 96–99. [Google Scholar]
- Smith, D.B.; Boileau, M.A.; Buan, L.D. A Self-Directed Home Biofeedback System for Women with Symptoms of Stress, Urge, and Mixed Incontinence. J. WOCN 2000, 27, 0240–0246. [Google Scholar] [CrossRef]
- Yoon, H.S.; Song, H.H.; Ro, Y.J. A Comparison of Effectiveness of Bladder Training and Pelvic Muscle Exercise on Female Urinary Incontinence. Int. J. Nurs. Stud. 2003, 40, 45–50. [Google Scholar] [CrossRef]
- Burgio, K.L.; Kraus, S.R.; Menefee, S.; Borello-France, D.; Corton, M.; Johnson, H.W.; Mallett, V.; Norton, P.; FitzGerald, M.P.; Dandreo, K.J.; et al. Behavioral Therapy to Enable Women with Urge Incontinence to Discontinue Drug Treatment: A Randomized Trial. Ann. Intern. Med. 2008, 149, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Lauti, M.; Herbison, P.; Hay-Smith, J.; Ellis, G.; Wilson, D. Anticholinergic Drugs, Bladder Retraining and Their Combination for Urge Urinary Incontinence: A Pilot Randomised Trial. Int. Urogynecol. J. 2008, 19, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Burgio, K.L.; Kraus, S.R.; Borello-France, D.; Chai, T.C.; Kenton, K.; Goode, P.S.; Xu, Y.; Kusek, J.W. The Effects of Drug and Behavior Therapy on Urgency and Voiding Frequency. Int. Urogynecol. J. 2010, 21, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Ketai, L.H.; Komesu, Y.M.; Schrader, R.M.; Rogers, R.G.; Sapien, R.E.; Dodd, A.B.; Mayer, A.R. Mind-Body (Hypnotherapy) Treatment of Women with Urgency Urinary Incontinence: Changes in Brain Attentional Networks. Am. J. Obstet. Gynecol. 2021, 224, 498.e1–498.e10. [Google Scholar] [CrossRef] [PubMed]
- Komesu, Y.M.; Schrader, R.M.; Rogers, R.G.; Sapien, R.E.; Mayer, A.R.; Ketai, L.H. Hypnotherapy or Medications: A Randomized Noninferiority Trial in Urgency Urinary Incontinent Women. Am. J. Obstet. Gynecol. 2020, 222, 159.e1–159.e16. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Newman, D.; Schwartz, T.A.; Zou, B.; Miller, J.; Palmer, M.H. Effects of Unsupervised Behavioral and Pelvic Floor Muscle Training Programs on Nocturia, Urinary Urgency, and Urinary Frequency in Postmenopausal Women: Secondary Analysis of a Randomized, Two-Arm, Parallel Design, Superiority Trial (TULIP Study). Maturitas 2021, 146, 42–48. [Google Scholar] [CrossRef]
- Pennisi, M.; Grasso-Leanza, F.; Panella, P.; Pepe, P. Rehabilitation therapy in the treatment of female urinary incontinence. Our experience with 121 patients. Minerva Urol. Nefrol. 1994, 46, 245–249. [Google Scholar]
- Rasero, L.; Mangani, L. La riabilitazione urologica per l’incontinenza urinaria femminile. Studio prospettico sull’utilizzo di tecniche riabilitative per il pavimento pelvico. Assist. Inferm. Ric. 2005, 24, 20–24. [Google Scholar]
- Jeffcoate, T.N.; Francis, W.J. Urgency Incontinence in the Female. Am. J. Obstet. Gynecol. 1966, 94, 604–618. [Google Scholar] [CrossRef]
- Minassian, V.A.; Drutz, H.P.; Al-Badr, A. Urinary Incontinence as a Worldwide Problem. Int. J. Gynecol. Obstet. 2003, 82, 327–338. [Google Scholar] [CrossRef]
- Magid, E. Evidence-Based Medicine. How to Practice and Teach EBM, 2nd Ed. David L. Sackett, Sharon E. Straus, W. Scott Richardson, William Rosenberg, and R. Brian Haynes. Edinburgh: Churchill Livingstone, 2000, 261 Pp. (plus One Compact Disc), $34.95. ISBN 0-443-06240-4. Clin. Chem. 2001, 47, 1747. [Google Scholar] [CrossRef]
- Bergh, A.-L.; Friberg, F.; Persson, E.; Dahlborg-Lyckhage, E. Registered Nurses’ Patient Education in Everyday Primary Care Practice: Managers’ Discourses. Glob. Qual. Nurs. Res. 2015, 2, 233339361559916. [Google Scholar] [CrossRef] [PubMed]
- Bokne, K.; Sjöström, M.; Samuelsson, E. Self-Management of Stress Urinary Incontinence: Effectiveness of Two Treatment Programmes Focused on Pelvic Floor Muscle Training, One Booklet and One Internet-Based. Scand. J. Prim. Health Care 2019, 37, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, J.C.; Avery, M.D. The 2012 American College of Nurse-Midwives Core Competencies for Basic Midwifery Practice: History and Revision. J. Midwifery Womens Health 2014, 59, 82–90. [Google Scholar] [CrossRef]
- Hung, H.-C.; Hsiao, S.-M.; Chih, S.-Y.; Lin, H.-H.; Tsauo, J.-Y. An Alternative Intervention for Urinary Incontinence: Retraining Diaphragmatic, Deep Abdominal and Pelvic Floor Muscle Coordinated Function. Man. Ther. 2010, 15, 273–279. [Google Scholar] [CrossRef]
| Quasi-Experimental Studies | Author and Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Pennisi et al., 1994 [53] | Y | U | N | Y | U | Y | Y | U | N | 44% | |
| Lagro-Janssen and van Weel, 1998 [43] | Y | N/A | Y | N | Y | Y | U | Y | Y | 67% | |
| Smith, Boileau and Buan, 2000 [45] | Y | Y | N/A | N | Y | Y | N/A | Y | Y | 67% | |
| Yoon, Song and Ro, 2003 [46] | Y | U | Y | Y | Y | U | Y | Y | Y | 78% | |
| Rasero and Mangani, 2005 [54] | Y | Y | N/A | N | Y | Y | N/A | U | N | 44% | |
| Kafri et al., 2007 [42] | Y | Y | N | N | Y | Y | Y | Y | Y | 78% | |
| Singh and Arya, 2011 [44] | Y | Y | N/A | N | Y | Y | N/A | Y | Y | 67% |
| Randomized Controlled Trials | Author and Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Burgio et al., 2008 [47] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 89% | |
| Lauti et al., 2008 [48] | Y | Y | Y | N | N | N | Y | Y | Y | Y | Y | Y | Y | 78% | |
| Burgio et al., 2010 [49] | Y | U | Y | U | U | U | Y | Y | Y | Y | Y | Y | Y | 78% | |
| Komesu et al., 2020 [51] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 89% | |
| Ketai et al., 2021 [50] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 89% | |
| Wadensten et al., 2021 [40] | Y | Y | Y | N | N/A | U | Y | Y | Y | Y | U | Y | Y | 67% | |
| Wu et al., 2021 [52] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 89% |
| # | Authors and Year | RCT/ Quasi-Experimental | Intervention 1 | Participants | Non-Pharmacological | Pharmacological | Intervention 2 | Non-Pharmacological | Pharmacological | Intervention 3 | Non-Pharmacological | Pharmacological | Efficacy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical | Psychological | Physical | Psychological | Physical | Psychological | |||||||||||
| 1 | Burgio et al., 2008 [47] | RCT | behavioral training + tolterodine 4 mg/day | A total of 307 women with urge-predominant incontinence randomly assigned in two groups in the study BE-DRI: combination therapy (drug therapy + behavioural training, n = 154, mean age 55.8) and drug therapy alone (n = 153, mean age 58.0). | ✓ | ✓ | ✓ | Tolterodine 4 mg/day | ✓ | ↓ in incontinence episodes was higher in the combination therapy group (69% vs. 58%) | ||||||
| 2 | Burgio et al., 2010 [49] | RCT | behavioral training + tolterodine 4 mg/day | A total of 307 women with mild and moderate urge and mixed incontinence. Mean age was 58 years old for the drug only group and 55.8 years old for the drug and behaviour group. A total of 153 were assigned to the “drug only” group and 154 to the “drug and behaviour” group. In both groups, treatment was imple- mented in four visits, at intervals of 2–3 weeks, over a period of 10 weeks. | ✓ | ✓ | ✓ | Tolterodine 4 mg/day | ✓ | ↓ in incontinence episodes was higher in the combination therapy group but with women with a mild urge urinary incontinence (p < 0.001) | ||||||
| 3 | Kafri et al., 2007 [42] | Quasi-experimental | behavioral training | A total of 44 women 18 years old or older, mean age: 55 [range 27–69] (mean age: 56.8 with SD = 8 for the medication group, 54.5 with SD = 9.7 for the rehabilitation group). Diagnosis of UUI and OAB in urodynamic testing. | ✓ | ✓ | Oxybutynin 5 mg/day | ✓ | ↓ in incontinence episodes was higher in the behavioral training group (p = 0.001) | |||||||
| 4 | Ketai et al., 2021 [50] | RCT | hypnotherapy | A total of 72 women with UUI were recruited from an academic urogynecology clinic and the community at large between March 2013 and April 2016. Hypnotherapy = 36 (mean age 54), Pharmacotherapy = 36 (mean age 57). | Oxybutynin 10 mg/day or tolterodine 4 mg/day | ✓ | = in incontinence episodes between groups (p = 0.001) | |||||||||
| 5 | Komesu et al., 2020 [51] | RCT | hypnotherapy | Women with non-neurogenic UUI for at least three months, Overactive Bladder-Awareness Tool scores ≥ eight, 18 and ≥ three UUI episodes per week. Mean age of the included women: 57.6 (SD = 12.77) hypnotherapy, 59.5 (SD = 10.30) pharmacotherapy. | Oxybutynin 10 mg/day or tolterodine 4 mg/day + counseling | ✓ | = in incontinence episodes between groups | |||||||||
| 6 | Lagro-Janssen and van Weel, 1998 [43] | Quasi-experimental | bladder training | A total of 88 women between 20 and 65 years (mean age: 50.6, SD = 10.4) presenting UI selected by 13 general practitioners. Type of incontinence: genuine stress: 56 (64%), MUI: 14 (16%), urge incontinence: 18 (21%). Severity of incontinence: severe: 28 (32%), moderate: 55 (62%), mild: 5 (6%). Mean parity 2.1 (SD = 1.3). Number using medication: 44 (50%). | ✓ | ↑ in incontinence episodes in the long term with a mean increase of 2.65 episodes for week | ||||||||||
| 7 | Lauti et al., 2008 [48] | RCT | bladder training + counseling | Women with predominant urge urinary incontinence experiencing at least monthly leakage and aged over 18 years. Mean age of the included women: 53.8 ± 14.8 (BRT), 63.9 ± 17.2 (DT), 47.6 ± 16.3 (COMBO). | ✓ | ✓ | Oxybutynin 2.5 mg/day | ✓ | bladder training and counseling + oxybutynin 2.5 mg/day | ✓ | ✓ | ✓ | =in incontinence episodes between groups | |||
| 8 | Pennisi et al., 1994 [53] | Quasi-experimental | physiotherapy | Women aged between 27 and 75 years old (mean age 51) with UI 121 women: 55 with SUI (20 treated with FKT, 35 with FKT + SEF), 20 with UUI (6 with FKT, 6 with FKT + Ditropan, 8 with FKT + SEF) and 46 with MUI (12 treated with FKT, 17 with FKT + Ditropan, 17 with FKT + SEF). | ✓ | Oxybutynin 5 mg/3 times per day | ✓ | physiotherapy + electric functional stimulation | ✓ | With physiotherapy, 83.3% of patients recovered, 26.7% improved. With the combination of physiotherapy and oxybutynin, 100% of patients recovered, and with the combination of physiotherapy and electric functional stimulation, 72.5% recovered and 37.5% improved their outcomes | ||||||
| 9 | Rasero and Mangani, 2005 [54] | Quasi-experimental | physiotherapy + electric functional stimulation | Women attending the pelvic floor rehabilitation clinic. No particular characteristics were required in order to access the rehabilitation program. Mean age of the included women: 57 years [range: 35-72]. | ✓ | A total of 14.3% of women improved their outcomes, 14.3% recovered in the physiotherapy + electric functional stimulation group | ||||||||||
| 10 | Singh and Arya, 2011 [44] | Quasi-experimental | bladder rehabilitation program | A total of 12 motivated, non-demented (Mini Mental State Examination > 24) and ambulatory, community-dwelling subjects were taken for the study between ages of 55 and -70 years. Mean age of the included women: 63.75 ± 5.17 years. UI or predominant MUI persisting for at least three months with frequency of at least 2 or more episodes per week. Experience of involuntary loss of urine associated with strong desire to void. | ✓ | ↓ in incontinence episodes (p = 0.000); statistical difference in pre–post tests of OABq and PIIQ with better scores (p = 0.000) | ||||||||||
| 11 | Smith, Boileau and Buan, 2000 [45] | Quasi-experimental | behavioral training + biofeedback | A total of 55 volunteer women with symptoms of urge, stress or mixed UI. The mean age was of 54 years [range 25–81]. Main characteristics of the participants: 37 were parous, 18 underwent a previous hysterectomy, 7 underwent a previous bladder surgery, 29 experienced both stress and urge symptoms and in 50%, symptoms lasted for more than 2 years. In total, 44 women completed the program. | ✓ | ✓ | ↓ in incontinence episodes after the intervention and mean continence severity (p < 0.001) | |||||||||
| 12 | Wadensten et al., 2021 [40] | RCT | behavioral training | A total of 123 women ≥ 18 years old with UUI or MUI and ≥2 leakages per week, access to a smartphone, and the ability to send and receive email. Mean age of the included women: 58 [range: 31–77] years. Treatment app (n = 60, 2 lost to follow-up) or the information app (control group, n = 63). Of these, 35 (28%) women had UUI, and 88 (72%) had MUI. | ✓ | ✓ | Information application | ✓ | ↓ in incontinence episodes in the intervention group (p < 0.001) | |||||||
| 13 | Wu et al., 2021 [52] | RCT | behavioral and pelvic floor muscle training (B-PFMT) | A total of 647 postmenopausal women with: nocturia, urinary urgency and urinary frequency. Mean age: 62.9 ± 5.7 | ✓ | ✓ | Informational DVD | ✓ | = in incontinence episodes between groups | |||||||
| 14 | Yoon, Song, and Ro, 2003 [46] | Quasi-experimental | bladder training | A total of 50 participants with urine loss or ≥ 14 voids every 48 h were recruited through public advertisements. All women were parous and aged 35–55. | ✓ | pelvic floor muscle training | ✓ | ↓ in incontinence episodes in the bladder training group (p < 0.01) | ||||||||
| # | Authors and Year | Frequency of Incontinence Episodes | Incontinence Symptoms | Nocturia | Voided Volume | Patient’s Satisfaction | Quality of Life | Distress | Brain Activation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Burgio et al., 2008 [47] | ✓ | ✓ | ✓ | ✓ | ||||
| 2 | Burgio et al., 2010 [49] | ✓ | |||||||
| 3 | Kafri et al., 2007 [42] | ✓ | ✓ | ✓ | |||||
| 4 | Ketai et al., 2021 [50] | ✓ | ✓ | ||||||
| 5 | Komesu et al., 2020 [51] | ✓ | |||||||
| 6 | Lagro-Janssen and van Weel, 1998 [43] | ✓ | |||||||
| 7 | Lauti et al., 2008 [48] | ✓ | ✓ | ✓ | |||||
| 8 | Pennisi et al., 1994 [53] | ✓ | |||||||
| 9 | Rasero and Mangani, 2005 [54] | ✓ | |||||||
| 10 | Singh and Arya, 2011 [44] | ✓ | |||||||
| 11 | Smith, Boileau and Buan, 2000 [45] | ✓ | |||||||
| 12 | Wadensten et al., 2021 [40] | ✓ | ✓ | ✓ | |||||
| 13 | Wu et al., 2021 [52] | ✓ | ✓ | ||||||
| 14 | Yoon, Song, and Ro, 2003 [46] | ✓ | ✓ | ✓ |
| # | Authors and Year | Adverse Events | Non-Pharmacological Intervention | Pharmacological Intervention | Worst in | Description |
|---|---|---|---|---|---|---|
| 1 | Burgio et al., 2008 [47] | YES | ✓ | ✓ | not reported | blurred vision, syncope, night sweats, stomach cramping, weakness, small bowel obstruction, allergic reaction (pruritus and rash) and tachycardia (in the combination therapy group) |
| 2 | Burgio et al., 2010 [49] | NOT REPORTED | ||||
| 3 | Kafri et al., 2007 [42] | YES | ✓ | ✓ | pharmacological | dry mouth and fatigue |
| 4 | Ketai et al., 2021 [50] | NOT REPORTED | ||||
| 5 | Komesu et al., 2020 [51] | YES | ✓ | ✓ | pharmacological | constipation, dyspepsia, dry eyes, dry mouth, voiding difficulties, urinary tract infection, falls, headache, back pain (in the pharmacological group), urinary tract infection, falls, headache, back pain (in the hypnotherapy group) |
| 6 | Lagro-Janssen and van Weel, 1998 [43] | NOT REPORTED | ||||
| 7 | Lauti et al., 2008 [48] | YES | ✓ | ✓ | pharmacological | dry mouth, headache, dizziness–vertigo, constipation, fatigue |
| 8 | Pennisi et al., 1994 [53] | NOT REPORTED | ||||
| 9 | Rasero and Mangani, 2005 [54] | NO | ||||
| 10 | Singh and Arya, 2011 [44] | NOT REPORTED | ||||
| 11 | Smith, Boileau and Buan, 2000 [45] | NOT REPORTED | ||||
| 12 | Wadensten et al., 2021 [40] | YES | ✓ | behavioral training | inguinal hernia, altered incontinence symptoms | |
| 13 | Wu et al., 2021 [52] | NOT REPORTED | ||||
| 14 | Yoon, Song, and Ro, 2003 [46] | NOT REPORTED |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trapani, S.; Villa, G.; Poliani, A.; Gnecchi, S.; Rosa, D.; Manara, D.F. Non-Pharmacological Management of Urge Urinary Incontinence in Women between 40 and 65 Years Old: A Systematic Review. Nurs. Rep. 2024, 14, 174-196. https://doi.org/10.3390/nursrep14010015
Trapani S, Villa G, Poliani A, Gnecchi S, Rosa D, Manara DF. Non-Pharmacological Management of Urge Urinary Incontinence in Women between 40 and 65 Years Old: A Systematic Review. Nursing Reports. 2024; 14(1):174-196. https://doi.org/10.3390/nursrep14010015
Chicago/Turabian StyleTrapani, Sara, Giulia Villa, Andrea Poliani, Silvia Gnecchi, Debora Rosa, and Duilio F. Manara. 2024. "Non-Pharmacological Management of Urge Urinary Incontinence in Women between 40 and 65 Years Old: A Systematic Review" Nursing Reports 14, no. 1: 174-196. https://doi.org/10.3390/nursrep14010015
APA StyleTrapani, S., Villa, G., Poliani, A., Gnecchi, S., Rosa, D., & Manara, D. F. (2024). Non-Pharmacological Management of Urge Urinary Incontinence in Women between 40 and 65 Years Old: A Systematic Review. Nursing Reports, 14(1), 174-196. https://doi.org/10.3390/nursrep14010015







