Quality of Life of Polish Patients with Lymphoma Treated Systemically
Abstract
:1. Introduction
- (1)
- How do the surveyed patients assess their quality of life and health as well as their physical health, psychological condition, social relations, and the environment?
- (2)
- How do demographic variables affect the respondents’ assessment of their quality of life?
- (3)
- How does the support received affect the respondents’ assessment of their quality of life?
- (4)
- How do the side effects of systemic treatment and comorbidities affect patients’ assessment of their quality of life?
2. Materials and Methods
2.1. Research Methods
2.2. Organisation and Research Area
2.3. Statistical Analysis
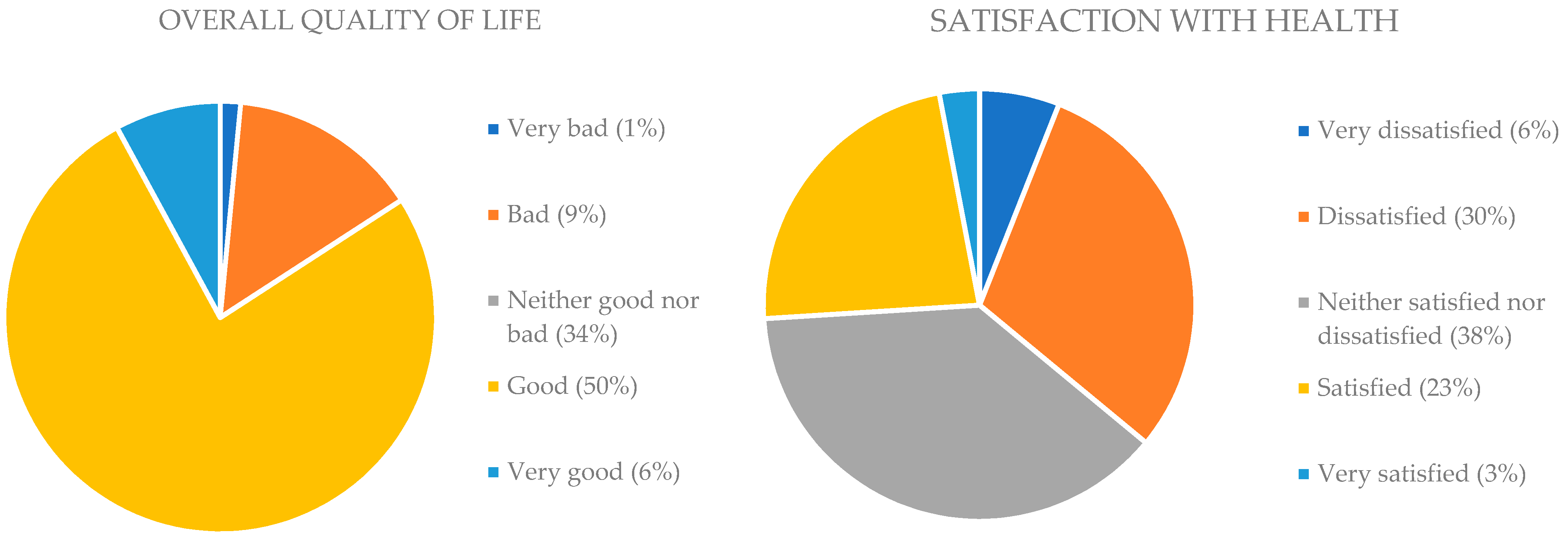
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Public Involvement Statement
Guidelines and Standards Statement
Acknowledgments
Conflicts of Interest
References
- Reports|Polish National Cancer Registry. Available online: https://onkologia.org.pl/en/report (accessed on 8 February 2023).
- Jiang, M.; Bennani, N.N.; Feldman, A.L. Lymphoma Classification Update: T-Cell Lymphomas, Hodgkin Lymphomas, and Histiocytic/Dendritic Cell Neoplasms. Expert Rev. Hematol. 2017, 10, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-W.; Balakrishna, J.P.; Pittaluga, S.; Jaffe, E.S. Diagnosis of Hodgkin Lymphoma in the Modern Era. Br. J. Haematol. 2019, 184, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M. Hodgkin Lymphoma: A 2020 Update on Diagnosis, Risk-stratification, and Management. Am. J. Hematol. 2020, 95, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Doyle-McClam, M.; Pugh, S.; Dudney, T.; McCormack, M.; Kravitz, J. Diagnostic and Treatment Dilemma during the Coronavirus Disease 2019 Pandemic: A Primary Pulmonary Lymphoma Presenting as a Cavitary Mass in a Patient with Coronavirus Disease 2019: A Case Report. J. Med. Case Rep. 2023, 17, 12. [Google Scholar] [CrossRef]
- Ansell, S.M.; Armitage, J.O. Management of Hodgkin Lymphoma. Mayo Clin. Proc. 2006, 81, 419–426. [Google Scholar] [CrossRef]
- Zahid, U.; Akbar, F.; Amaraneni, A.; Husnain, M.; Chan, O.; Riaz, I.B.; McBride, A.; Iftikhar, A.; Anwer, F. A Review of Autologous Stem Cell Transplantation in Lymphoma. Curr. Hematol. Malig. Rep. 2017, 12, 217–226. [Google Scholar] [CrossRef]
- Khan, N.; Moskowitz, A.J. Where Do the New Drugs Fit in for Relapsed/Refractory Hodgkin Lymphoma? Curr. Hematol. Malig. Rep. 2017, 12, 227–233. [Google Scholar] [CrossRef]
- Van Dijck, R.; Doorduijn, J.K.; Bromberg, J.E.C. The Role of Rituximab in the Treatment of Primary Central Nervous System Lymphoma. Cancers 2021, 13, 1920. [Google Scholar] [CrossRef]
- Toukam, M.; Boni, J.P.; Hamadani, M.; Caimi, P.F.; Cruz, H.G.; Wuerthner, J. Exposure–Response Analysis of Camidanlumab Tesirine in Patients with Relapsed or Refractory Classical Hodgkin Lymphoma and Non-Hodgkin Lymphoma. Cancer Chemother. Pharmacol. 2023, 91, 1–12. [Google Scholar] [CrossRef]
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.-H.; Bartlett, N.L. Hodgkin Lymphoma. Nat. Rev. Dis. Primers 2020, 6, 61. [Google Scholar] [CrossRef]
- Ariestine, D.A.; Sari, N.K.; Rinaldi, I.; Abdullah, M. Quality of Life in Older Survivors of Non-Hodgkin’s Lymphoma Who Received Chemotherapy and Related Factors. J. Geriatr. Oncol. 2021, 12, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Lekdamrongkul, P.; Pongthavornkamol, K.; Molassiotis, A.; Sriyuktasuth, A.; Siritanaratkul, N.; Chansatitporn, N. Exploring Health-Related Quality of Life among Non-Hodgkin’s Lymphoma Survivors after Completion of Primary Treatment: A Cross-Sectional Study in Thailand. Support Care Cancer 2021, 29, 6511–6522. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H. Quality of Life: Principles of the Clinical Paradigm. J. Psychosoc. Oncol. 1990, 8, 171–185. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S. Measuring Quality of Life Today: Methodological Aspects. Oncology 1990, 4, 29–38, discussion 69. [Google Scholar] [PubMed]
- Trzebiatowski, J. Quality of Life in the Perspective of Social and Medical Sciences–Classification of Definitions. Hygeia Public Health 2011, 46, 25–31. [Google Scholar]
- Wołowicka, L. Quality of Life in Medical Sciences; Academic Publishing Department of the Medical University of Karol Marcinkowski: Poznan, Poland, 2001. [Google Scholar]
- McCaffrey, N.; Kaambwa, B.; Currow, D.C.; Ratcliffe, J. Health-Related Quality of Life Measured Using the EQ-5D–5L: South Australian Population Norms. Health Qual. Life Outcomes 2016, 14, 133. [Google Scholar] [CrossRef]
- EORTC Quality of Life Website-EORTC-Quality of Life: EORTC–Quality of Life. Available online: https://qol.eortc.org/ (accessed on 11 February 2023).
- Jaracz, K.; Kalfoss, M.; Gorna, K.; Bączyk, G. Quality of Life in Polish Respondents: Psychometric Properties of the Polish WHOQOL–Bref. Scand. J. Caring Sci. 2006, 20, 251–260. [Google Scholar] [CrossRef]
- Leppert, W.; Forycka, M.; de Walden-Gałuszko, K.; Majkowicz, M.; Buss, T. Quality of Life Assessment in Cancer Patients–Recommendations for the Staff of Oncology and Palliative Care Units. Psychoonkologia 2014, 18, 17–29. [Google Scholar]
- Zhang, J.; Xiao, S.; Shi, L.; Xue, Y.; Zheng, X.; Dong, F.; Xue, B.; Zhang, C. Differences in Health-Related Quality of Life and Its Associated Factors Among Older Adults in Urban and Rural Areas. RMHP 2022, 15, 1447–1457. [Google Scholar] [CrossRef]
- Ślusarska, B.; Nowicki, G.J.; Serwata, M.; Zboina, B.; Łuczyk, M.; Szadowska-Szlachetka, Z. Level of Disease Acceptance and Quality of Life in People with Lymphoma. Palliat. Med. 2016, 8, 88–95. [Google Scholar]
- Ma, Y.; He, B.; Jiang, M.; Yang, Y.; Wang, C.; Huang, C.; Han, L. Prevalence and Risk Factors of Cancer-Related Fatigue: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2020, 111, 103707. [Google Scholar] [CrossRef]
- Inglis, J.E.; Lin, P.-J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; Castillo, D.A.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef]
- Belloni, S.; Bonucci, M.; Arrigoni, C.; Dellafiore, F.; Caruso, R. A Systematic Review of Systematic Reviews and a Pooled Meta-Analysis on Complementary and Integrative Medicine for Improving Cancer-Related Fatigue. Clin. Ther. 2022, 45, e54–e73. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Próchnicki, M.; Rudzki, S.; Laskowska, B.; Brudniak, J. Quality of Life of Cancer Patients Treated with Chemotherapy. Int. J. Environ. Res. Public Health 2020, 17, 6938. [Google Scholar] [CrossRef] [PubMed]
- Rosińczuk, J.; Kołtuniuk, A.; Lech, K. Patients’ Quality of Life during Treatment with Cytostatic Drugs in Hematologic Cancers. Ann. Acad. Med. Siles. 2016, 70, 184–195. [Google Scholar] [CrossRef]
- Milaniak, I.; Marczewska, E. The Assessment of Influence of Fatigue on Quality of Life Lung Cancer Patients. Nurs. Probl. 2014, 22, 327–332. [Google Scholar]
- Zielińska-Więczkowska, H.; Betłakowski, J. Measurement and Assessment of Social Support in Hospitalized Patients Undergoing Chemotherapy. Contemp. Oncol. Współczesna Onkol. 2010, 14, 141–144. [Google Scholar] [CrossRef]
- Pasek, M.; Goździalska, A.; Jochymek, M.; Caruso, R. Social Support in a Cancer Patient-Informal Caregiver Dyad: A Scoping Review. Cancers 2023, 15, 1754. [Google Scholar] [CrossRef]
| Variables | N Respondents | % Respondents | |
|---|---|---|---|
| Sex | Female | 63 | 76.82 |
| Male | 19 | 23.18 | |
| Age (years) | 20–30 | 20 | 24.40 |
| 31–40 | 23 | 28.05 | |
| 41–50 | 14 | 17.07 | |
| 51–60 | 7 | 8.54 | |
| 61–70 | 14 | 17.07 | |
| Over 70 | 4 | 4.87 | |
| Education | Preliminary | 4 | 4.87 |
| Vocational | 8 | 9.75 | |
| Secondary | 25 | 30.49 | |
| Higher | 45 | 54.89 | |
| Place of residence | City of more than 50,000 residents | 44 | 53.66 |
| City of fewer than 50,000 residents | 22 | 26.83 | |
| Village | 16 | 19.51 | |
| Work activity | Professional work | 23 | 28.05 |
| Sick leave | 23 | 28.05 | |
| Retirement/pension | 23 | 28.05 | |
| Other | 13 | 15.85 | |
| Family status | Lives with a family | 60 | 73.17 |
| Lives with a partner | 13 | 15.85 | |
| Lives alone | 9 | 10.98 |
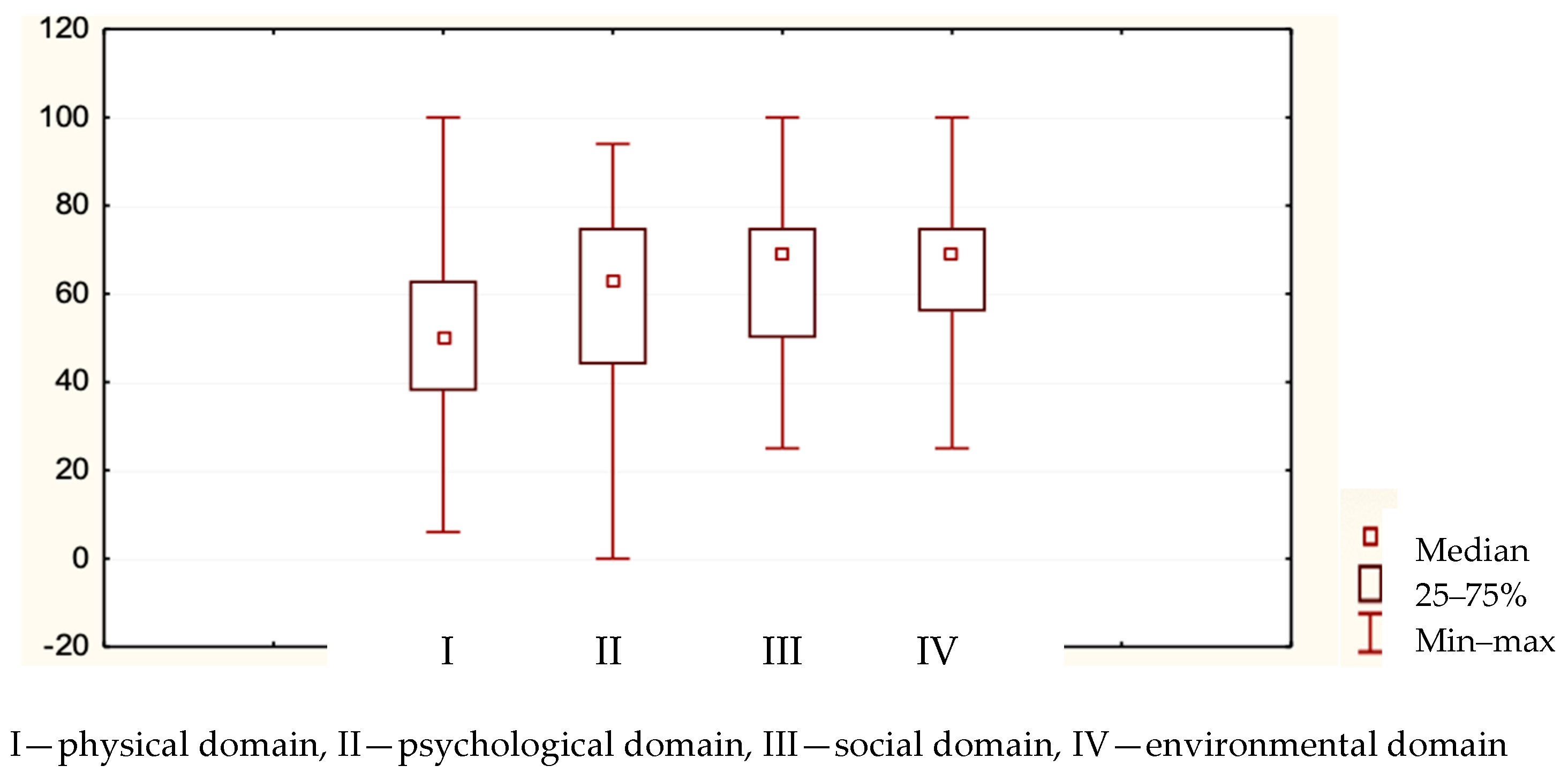
| WHOQOL Domains | Average Rank | Sum of Ranks | Mean [0–100] | Standard Deviation |
|---|---|---|---|---|
| Physical | 1.80 | 147.5 | 51.32 | 19.17 |
| Psychological | 2.58 | 211.5 | 60.38 | 20.18 |
| Social | 2.73 | 224.0 | 62.82 | 17.86 |
| Environmental | 2.89 | 237.0 | 64.30 | 16.05 |
| Age and WHOQOL Domains | N Significant | Spearman’s Rho | T (n − 2) | p |
|---|---|---|---|---|
| physical | 82 | −0.03 | −0.25 | 0.80 |
| psychological | 82 | 0.54 | 0.49 | 0.68 |
| social | 82 | 0.061 | 0.54 | 0.59 |
| environmental | 82 | 0.068 | 0.61 | 0.54 |
| Caregivers | Mean | Median | Min | Max | Lower Quartile | Higher Quartile | Standard Deviation |
|---|---|---|---|---|---|---|---|
| Family | 6.16 | 7 | 1 | 7 | 6 | 7 | 1.48 |
| Friends | 5.32 | 6 | 1 | 7 | 4 | 7 | 1.77 |
| Institutions | 1.45 | 1 | 1 | 7 | 1 | 1 | 1.12 |
| Hematology staff | 4.55 | 5 | 1 | 7 | 4 | 6 | 1.83 |
| Other people | 2.63 | 2 | 1 | 7 | 1 | 4 | 1.84 |
| Caregivers Psychological Domain | N Significant | Spearman’s Rho | T (n − 2) | p |
|---|---|---|---|---|
| Family | 82 | 0.38 | 3.63 | 0.00 |
| Friends | 82 | 0.35 | 3.36 | 0.00 |
| Ward staff | 82 | 0.41 | 4.03 | 0.00 |
| Institutions | 82 | 0.01 | −0.08 | 0.93 |
| Other | 82 | 0.33 | 3.12 | 0.00 |
| Side Effects during Hospitalization | Never | Sometimes | Often | Very Often | ||||
|---|---|---|---|---|---|---|---|---|
| [n] | [%] | [n] | [%] | [n] | [%] | [n] | [%] | |
| Weakness | 0 | 0 | 24 | 29 | 32 | 39 | 26 | 32 |
| Diarrhea | 51 | 62 | 22 | 27 | 8 | 10 | 1 | 1 |
| Constipation | 23 | 28 | 36 | 44 | 14 | 17 | 9 | 11 |
| Nausea | 24 | 29 | 36 | 44 | 11 | 13 | 11 | 13 |
| Vomiting | 47 | 57 | 26 | 32 | 5 | 6 | 4 | 5 |
| No appetite | 24 | 29 | 41 | 50 | 13 | 16 | 4 | 5 |
| Skin lesions | 39 | 48 | 29 | 35 | 8 | 10 | 6 | 7 |
| Hair loss | 8 | 10 | 13 | 16 | 25 | 30 | 36 | 44 |
| Side Effects at Home | Never | Sometimes | Often | Very Often | ||||
|---|---|---|---|---|---|---|---|---|
| [n] | [%] | [n] | [%] | [n] | [%] | [n] | [%] | |
| Weakness | 3 | 4 | 25 | 30 | 27 | 33 | 27 | 33 |
| Diarrhea | 51 | 62 | 22 | 27 | 8 | 10 | 1 | 1 |
| Constipation | 29 | 35 | 31 | 38 | 15 | 18 | 7 | 9 |
| Nausea | 28 | 34 | 29 | 35 | 15 | 18 | 10 | 12 |
| Vomiting | 50 | 61 | 21 | 26 | 7 | 9 | 4 | 5 |
| No appetite | 29 | 35 | 42 | 51 | 9 | 11 | 2 | 2 |
| Weight loss | 39 | 48 | 27 | 33 | 13 | 16 | 3 | 4 |
| Hair loss | 14 | 17 | 15 | 18 | 27 | 33 | 26 | 32 |
| Memory problems | 23 | 28 | 24 | 29 | 23 | 28 | 12 | 15 |
| Insomnia | 20 | 24 | 35 | 43 | 17 | 21 | 10 | 12 |
| Limited mobility | 23 | 28 | 30 | 37 | 19 | 23 | 10 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasek, M.; Biel, J.; Goździalska, A.; Jochymek, M. Quality of Life of Polish Patients with Lymphoma Treated Systemically. Nurs. Rep. 2023, 13, 1421-1431. https://doi.org/10.3390/nursrep13040119
Pasek M, Biel J, Goździalska A, Jochymek M. Quality of Life of Polish Patients with Lymphoma Treated Systemically. Nursing Reports. 2023; 13(4):1421-1431. https://doi.org/10.3390/nursrep13040119
Chicago/Turabian StylePasek, Małgorzata, Janina Biel, Anna Goździalska, and Małgorzata Jochymek. 2023. "Quality of Life of Polish Patients with Lymphoma Treated Systemically" Nursing Reports 13, no. 4: 1421-1431. https://doi.org/10.3390/nursrep13040119
APA StylePasek, M., Biel, J., Goździalska, A., & Jochymek, M. (2023). Quality of Life of Polish Patients with Lymphoma Treated Systemically. Nursing Reports, 13(4), 1421-1431. https://doi.org/10.3390/nursrep13040119








