Evidence-Informed Development of a Bundle for Peripheral Intravenous Catheterization in Portugal: A Delphi Consensus Study
Abstract
:1. Introduction
2. Materials and Methods
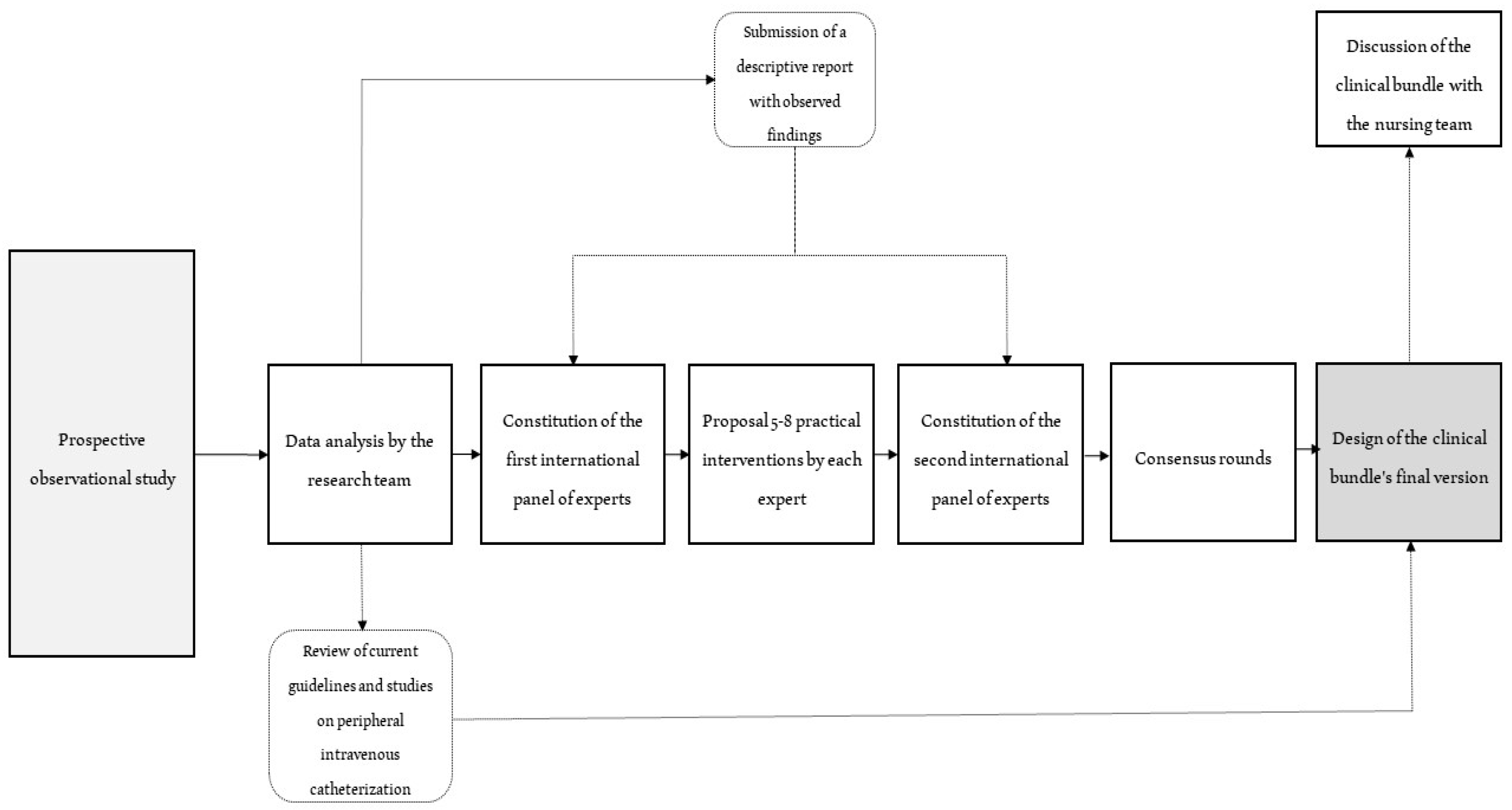
2.1. Study Design
2.2. Study Procedures
2.3. Participants
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Delphi Panel: Round One
3.2. Delphi Panel: Rounds Two and Three
3.3. Introducing and Reviewing the Bundle in a Clinical Setting
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandrou, E.; Ray-Barruel, G.; Carr, P.J.; Frost, S.A.; Inwood, S.; Higgins, N.; Lin, F.; Alberto, L.; Mermel, L.; Rickard, C.M.; et al. Use of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes Worldwide. J. Hosp. Med. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Mavillard, I.; Rodríguez-Calero, M.Á.; de Pedro-Gómez, J.; Parra-García, G.; Fernández-Fernández, I.; Castro-Sánchez, E. Incidence of Peripheral Intravenous Catheter Failure among Inpatients: Variability between Microbiological Data and Clinical Signs and Symptoms. Antimicrob. Resist. Infect. Control 2019, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Marsh, N.; Webster, J.; Ullman, A.J.; Mihala, G.; Cooke, M.; Chopra, V.; Rickard, C.M. Peripheral Intravenous Catheter Non-Infectious Complications in Adults: A Systematic Review and Meta-Analysis. J. Adv. Nurs. 2020, 76, 3346–3362. [Google Scholar] [CrossRef]
- Blanco-Mavillard, I.; de Pedro-Gómez, J.E.; Rodríguez-Calero, M.Á.; Bennasar-Veny, M.; Parra-García, G.; Fernández-Fernández, I.; Bujalance-Hoyos, J.; Moya-Suárez, A.B.; Cobo-Sánchez, J.L.; Ferrer-Cruz, F.; et al. Multimodal Intervention for Preventing Peripheral Intravenous Catheter Failure in Adults (PREBACP): A Multicentre, Cluster-Randomised, Controlled. Trial. Lancet Haematol 2021, 8, e637–e647. [Google Scholar] [CrossRef]
- Santos-Costa, P.; Sousa, L.; Torre-Montero, J.; Salgueiro-Oliveira, A.; Parreira, P.; Vieira, M.; Graveto, J. Translation, cultural adaptation, and validation of the Venous InternationalAssessment Scale to European Portuguese. Rev. Enferm. Ref. 2021, 5, e20135. [Google Scholar] [CrossRef]
- Santos-Costa, P.; Sousa, L.B.; van Loon, F.H.J.; Salgueiro-Oliveira, A.; Parreira, P.; Vieira, M.; Graveto, J. Translation and Validation of the Modified A-DIVA Scale to European Portuguese: Difficult Intravenous Access Scale for Adult Patients. Int. J. Environ. Res. Public Health 2020, 17, 7552. [Google Scholar] [CrossRef]
- Emergency Care Research Institute. Top 10 Patient Safety Concerns 2021; Emergency Care Research Institute: Plymouth Meeting, PA, USA, 2021; pp. 1–26. [Google Scholar]
- Loveday, H.P.; Wilson, J.A.; Pratt, R.J.; Golsorkhi, M.; Tingle, A.; Bak, A.; Browne, J.; Prieto, J.; Wilcox, M. Epic3: National Evidence-Based Guidelines for Preventing Healthcare-Associated Infections in NHS Hospitals in England. J. Hosp. Infect. 2014, 86, S1–S70. [Google Scholar] [CrossRef]
- Keogh, S.; Shelverton, C.; Flynn, J.; Mihala, G.; Mathew, S.; Davies, K.; Marsh, N.; Rickard, C. Implementation and Evaluation of Peripheral Intravascular Catheter Flushing Guidelines: A Stepped Wedge Cluster Randomised Trial. BMC Med. 2020, 18, 252. [Google Scholar] [CrossRef]
- Takashima, M.; Cooke, M.; DeVries, M.; Kleidon, T.M.; Alexandrou, E.; Chopra, V.; Rickard, C.M. An Implementation Framework for the Clinically Indicated Removal Policy for Peripheral Intravenous Catheters. J. Nurs. Care Qual. 2020; publish ahead of print. [Google Scholar] [CrossRef]
- Pittiruti, M.; Scoppettuolo, G. Raccomandazioni Gavecelt 2021 per la Indicazione, L’impianto e la Gestione dei Dispositivi per Accesso Venoso. 2021. Available online: https://gavecelt.it/nuovo/sites/default/files/uploads/Raccomandazioni%20GAVeCeLT%202021%20-%20v.2.0.pdf (accessed on 1 May 2022).
- Cortés Rey, N.; Pinelli, F.; van Loon, F.H.J.; Caguioa, J.; Munoz Mozas, G.; Piriou, V.; Teichgräber, U.; Lepelletier, D.; Mussa, B. The State of Vascular Access Teams: Results of a European Survey. Int. J. Clin. Pract. 2021, 75, e14849. [Google Scholar] [CrossRef]
- Edwards, M.; Rodway, A.D.; Ahmed, I.G.; Harvey, R.A.; Foad, T.S.; El Sakka, K.M. A Modular Vascular Access Training Program for Higher Surgical Trainees. J. Vasc. Access 2018, 19, 162–166. [Google Scholar] [CrossRef]
- Morrow, S.; DeBoer, E.; Potter, C.; Gala, S.; Alsbrooks, K. Vascular Access Teams: A Global Outlook on Challenges, Benefits, Opportunities, and Future Perspectives. J. Assoc. Vasc. Access 2022, 27, 8–18. [Google Scholar] [CrossRef]
- Furtado, L.C.d.R. Incidence and Predisposing Factors of Phlebitis in a Surgery Department. Br. J. Nurs. 2011, 20, S16–S18, S20, S22. [Google Scholar] [CrossRef] [PubMed]
- Santos-Costa, P.; Sousa, L.; Marques, I.; Oliveira, A.; Parreira, P.; Vieira, M.; Graveto, J. Studies carried out in Portugal in the area of peripheral venous catheterization: Scoping review protocol. Rev. Enferm. Ref. 2020, 5, e20004. [Google Scholar] [CrossRef]
- Salgueiro-Oliveira, A.; Bernardes, R.A.; Adriano, D.; Serambeque, B.; Santos-Costa, P.; Sousa, L.B.; Gama, F.; Barroca, R.; Braga, L.M.; Graveto, J.; et al. Peripherally Inserted Central Catheter Placement in a Cardiology Ward: A Focus Group Study of Nurses’ Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 7618. [Google Scholar] [CrossRef]
- Parreira, P.; Vicente, R.; Bernardes, R.A.; Sousa, L.B.; Serambeque, B.; Costa, P.; Braga, L.M.; Mónico, L.; Salgueiro-Oliveira, A. The Flushing Procedure in Nursing Practices: A Cross-Sectional Study with Portuguese and Brazilian Nurses. Heliyon 2020, 6, e04579. [Google Scholar] [CrossRef]
- Crowell, J.; O’Neil, K.; Drager, L. Project HANDS: A Bundled Approach to Increase Short Peripheral Catheter Dwell Time. J. Infus. Nurs. 2017, 40, 274–280. [Google Scholar] [CrossRef]
- Resar, R.; Griffin, F.; Haraden, C.; Nolan, T.W. Using Care Bundles to Improve Health Care Quality; IHI Innovation Series; Institute for Healthcare Improvement: Cambridge, MA, USA, 2012; pp. 1–18. [Google Scholar]
- Duncan, M.; Warden, P.; Bernatchez, S.F.; Morse, D. A Bundled Approach to Decrease the Rate of Primary Bloodstream Infections Related to Peripheral Intravenous Catheters. J. Assoc. Vasc. Access 2018, 23, 15–22. [Google Scholar] [CrossRef]
- Ray-Barruel, G.; Xu, H.; Marsh, N.; Cooke, M.; Rickard, C.M. Effectiveness of Insertion and Maintenance Bundles in Preventing Peripheral Intravenous Catheter-Related Complications and Bloodstream Infection in Hospital Patients: A Systematic Review. Infect. Dis. Health 2019, 24, 152–168. [Google Scholar] [CrossRef] [Green Version]
- Steere, L.; Ficara, C.; Davis, M.; Moureau, N. Reaching One Peripheral Intravenous Catheter (PIVC) Per Patient Visit With Lean Multimodal Strategy: The PIV5RightsTM Bundle. J. Assoc. Vasc. Access 2019, 24, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Schults, J.; Kleidon, T.; Chopra, V.; Cooke, M.; Paterson, R.; Ullman, A.J.; Marsh, N.; Ray-Barruel, G.; Hill, J.; Devrim, İ.; et al. International Recommendations for a Vascular Access Minimum Dataset: A Delphi Consensus-Building Study. BMJ Qual. Saf. 2021, 30, 722–730. [Google Scholar] [CrossRef]
- McIntyre, D.; Coyer, F.; Bonner, A. Identifying Nurse Sensitive Indicators Specific to Haemodialysis Nursing: A Delphi Approach. Collegian 2020, 27, 75–81. [Google Scholar] [CrossRef]
- Primdahl, S.C.; Todsen, T.; Clemmesen, L.; Knudsen, L.; Weile, J. Rating Scale for the Assessment of Competence in Ultrasound-Guided Peripheral Vascular Access—A Delphi Consensus Study. J. Vasc. Access 2016, 17, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Santos-Costa, P.; Paiva-Santos, F.; Sousa, L.B.; Bernardes, R.A.; Ventura, F.; Fearnley, W.D.; Salgueiro-Oliveira, A.; Parreira, P.; Vieira, M.; Graveto, J. Nurses’ Practices in the Peripheral Intravenous Catheterization of Adult Oncology Patients: A Mix-Method Study. J. Pers. Med. 2022, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Gorski, L.A.; Hadaway, L.; Hagle, M.E.; Broadhurst, D.; Clare, S.; Kleidon, T.; Meyer, B.M.; Nickel, B.; Rowley, S.; Sharpe, E.; et al. Infusion Therapy Standards of Practice, 8th Edition. J. Infus. Nurs. 2021, 44, S1–S224. [Google Scholar] [CrossRef]
- Moureau, N.L. (Ed.) Vessel Health and Preservation: The Right Approach for Vascular Access; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-03148-0. [Google Scholar]
- Royal College of Nursing. Standards for Infusion Therapy; Royal College of Nursing: London, UK, 2016. [Google Scholar]
- Avella, J. Delphi Panels: Research Design, Procedures, Advantages, and Challenges. Int. J. Dr. Stud. 2016, 11, 305–321. [Google Scholar] [CrossRef]
- Jünger, S.; Payne, S.A.; Brine, J.; Radbruch, L.; Brearley, S.G. Guidance on Conducting and REporting DElphi Studies (CREDES) in Palliative Care: Recommendations Based on a Methodological Systematic Review. Palliat. Med. 2017, 31, 684–706. [Google Scholar] [CrossRef] [Green Version]
- Youngblut, J.M.; Brooten, D. Evidence-Based Nursing Practice: Why Is It Important? AACN Clin. Issues Adv. Pract. Acute Crit. Care 2001, 12, 468–476. [Google Scholar] [CrossRef]
- Mackey, A.; Bassendowski, S. The History of Evidence-Based Practice in Nursing Education and Practice. J. Prof. Nurs. 2017, 33, 51–55. [Google Scholar] [CrossRef]
- Furtado, L.C.d.R. Maintenance of Peripheral Venous Access and Its Impact on the Development of Phlebitis: A Survey of 186 Catheters in a General Surgery Department in Portugal. J. Infus. Nurs. 2011, 34, 382–390. [Google Scholar] [CrossRef]
- Oliveira, A.; Costa, P.; Graveto, J.; Costa, F.; Osório, N.; Cosme, A.; Parreira, P. Práticas dos enfermeiros na cateterização intravenosa: Um estudo descritivo. Rev. Enferm. Ref. 2019, 4, 111–120. [Google Scholar] [CrossRef]
- Silveira, S. Prevenção Da Infeção Associada Aos Cuidados de Saúde: Bundle Do Cateterismo Venoso Periférico. Master’s Thesis, Escola Superior de Enfermagem S. João de Deus, Universidade de Évora, Évora, Portugal, 2018. [Google Scholar]
- Kuhne, G.W.; Quigley, B.A. Understanding and Using Action Research in Practice Settings. New Dir. Adult Contin. Educ. 1997, 1997, 23–40. [Google Scholar] [CrossRef]
- Larsen, E.N.; Byrnes, J.; Marsh, N.; Rickard, C.M. Patient-Reported Outcome and Experience Measures for Peripheral Venous Catheters: A Scoping Review Protocol. Br. J. Nurs. 2021, 30, S30–S35. [Google Scholar] [CrossRef] [PubMed]

| Variables | Specialists | |
|---|---|---|
| Round 1 (n = 7) | Rounds 2–3 (n = 7) | |
| Age | M = 45 years (min. 33–max. 64) | M = 44 years (min. 37–max. 55) |
| Sex | ||
| Male | 3 (42.9%) | 2 (28.6%) |
| Female | 4 (57.1%) | 5 (71.4%) |
| Scientific Background | ||
| Medicine | 1 (14.3%) | 2 (28.6%) |
| Nursing | 6 (85.7%) | 5 (71.4%) |
| Highest academic title | ||
| Master’s Degree | 3 (42.9%) | 3 (42.9%) |
| PhD | 4 (57.1%) | 4 (57.1%) |
| Professional experience | M = 21 years (min. 12–max. 45) | M = 23 years (min. 14–max. 39) |
| Current professional setting | ||
| Clinical setting | 3 (42.9%) | 3 (42.9%) |
| Academia and/or research lab | 3 (42.9%) | 4 (57.1%) |
| Independent Consultant | 1 (14.3%) | 0 (0%) |
| Recommendations (Categorized) | Round 1 | Round 2 | Round 3 |
|---|---|---|---|
| Patient involvement | 6 | EII 1 M = 5.0 (IQR = 1.0) RoA = 71.4% | EII 1 M = 6.0 (IQR = 1.0) RoA = 100% |
| Patient education | 3 | ||
| Risk assessment | 4 | EII 2 M = 4.0 (IQR = 2.0) RoA = 57.1% | |
| Skin antisepsis | 5 | EII 3 M = 5.0 (IQR = 1.25) RoA = 57.1% | EII 2 M = 6.0 (IQR = 2.0) RoA = 85.7% |
| Antiseptic type and use | 1 | EII 4 M = 2.0 (IQR: 2.25) RoA: 28.6% | |
| Tourniquet type and use | 1 | EII 5 M = 2.0 (IQR = 1.25) RoA: 28.6% | |
| Aseptic no-touch technique | 5 | EII 6 M = 4.0 (IQR = 1.25) RoA = 57.1% | |
| Catheter flushing | 4 | EII 7 M = 4.0 (IQR = 2.25) RoA: 57.1% | EII 4 M = 6.0 (IQR = 1.0) RoA = 85.7% |
| Catheter lock | 1 | ||
| Flushing solution and technique | 2 | EII 8 M = 3.5 (IQR = 1.0) RoA: 42.9% | |
| Use of vein-locating technology | 1 | EII 9 M = 2.0 (IQR = 1.25) RoA: 28.6% | Integrated into EII 1 |
| Hand hygiene | 2 | EII 10 M = 2.0 (IQR = 1.25) RoA: 28.6% | Integrated into EII 2 |
| Glove use | 1 | EII 11 M = 2.0 (IQR = 1.0) RoA: 14.3% | |
| Catheter dressing | 3 | EII 12 M = 4.0 (IQR = 1.25) RoA = 57.1% | EII 3 M = 6.0 (IQR = 1.0) RoA = 71.4% |
| Catheter securement | 2 | ||
| Catheter maintenance (overall) | 3 | EII 13 M = 3.5 (IQR = 1.0) RoA = 57.1% | EII 5 M = 6.0 (IQR = 2.0) RoA = 85.7% |
| Catheter surveillance (complications) | 3 | EII 14 M = 4.0 (IQR = 1.25) RoA = 71.4% | |
| Team communication & collaboration | 1 | EII 15 M = 2.0 (IQR = 0.25) RoA = 0% | Excluded |
| Insertion and maintenance experts | 1 | EII 16 M = 2.0 (IQR = 0.25) RoA: 0% | Excluded |
| Total items | 49 | 16 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Costa, P.; Paiva-Santos, F.; Sousa, L.B.; Bernardes, R.A.; Ventura, F.; Salgueiro-Oliveira, A.; Parreira, P.; Vieira, M.; Graveto, J. Evidence-Informed Development of a Bundle for Peripheral Intravenous Catheterization in Portugal: A Delphi Consensus Study. Nurs. Rep. 2022, 12, 498-509. https://doi.org/10.3390/nursrep12030047
Santos-Costa P, Paiva-Santos F, Sousa LB, Bernardes RA, Ventura F, Salgueiro-Oliveira A, Parreira P, Vieira M, Graveto J. Evidence-Informed Development of a Bundle for Peripheral Intravenous Catheterization in Portugal: A Delphi Consensus Study. Nursing Reports. 2022; 12(3):498-509. https://doi.org/10.3390/nursrep12030047
Chicago/Turabian StyleSantos-Costa, Paulo, Filipe Paiva-Santos, Liliana B. Sousa, Rafael A. Bernardes, Filipa Ventura, Anabela Salgueiro-Oliveira, Pedro Parreira, Margarida Vieira, and João Graveto. 2022. "Evidence-Informed Development of a Bundle for Peripheral Intravenous Catheterization in Portugal: A Delphi Consensus Study" Nursing Reports 12, no. 3: 498-509. https://doi.org/10.3390/nursrep12030047
APA StyleSantos-Costa, P., Paiva-Santos, F., Sousa, L. B., Bernardes, R. A., Ventura, F., Salgueiro-Oliveira, A., Parreira, P., Vieira, M., & Graveto, J. (2022). Evidence-Informed Development of a Bundle for Peripheral Intravenous Catheterization in Portugal: A Delphi Consensus Study. Nursing Reports, 12(3), 498-509. https://doi.org/10.3390/nursrep12030047










