Recovery of Health and Wellbeing in Rural Cancer Survivors Following Primary Treatment: Analysis of UK Qualitative Interview Data
Abstract
:1. Introduction
1.1. Research Questions
- What are the specific challenges and opportunities faced by rural cancer survivors post-treatment?
- How does residing in a rural area influence recovery from cancer?
1.2. Rurality and Cancer Survivorship
2. Methods
2.1. Study Design
2.1.1. Interviewing
2.1.2. Theoretical Framework
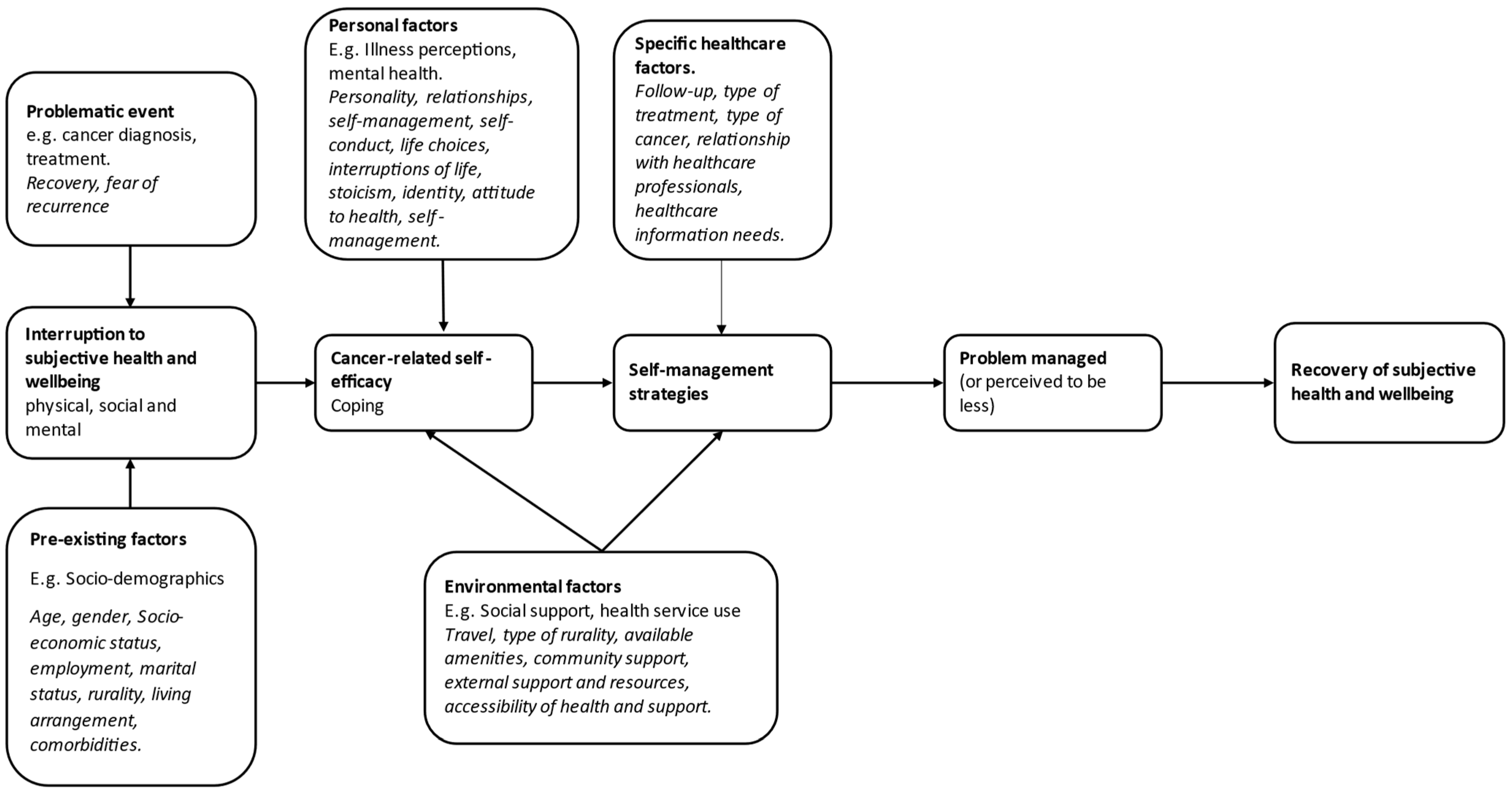
2.2. Conceptual Framework
2.3. Ethical Considerations
2.4. Data Analysis
3. Results
3.1. Demographics
3.2. Results in Relation to the Recovery of Health and Wellbeing in Cancer Survivorship Framework
3.2.1. Problematic Events
‘Then I had to wait three weeks for the pathology; and when I went back to see her, she said to me that it was a cancer. I just fell apart then because all I had been told was that it couldn’t be a cancer because you have had it so long’.(Female, 68)
‘[I] tried doing overnight feeds, not very successful. I found that psychologically, I found it very difficult to deal with’ (Male, 62). ‘[due to the chemotherapy] my feet are dodgy and my balance is not very good’.(Female, 81)
3.2.2. Pre-Existing Factors
‘[they] are lucky because we are pretty comfortable financially but how on earth someone who’s got what I’ve got [myeloma] that’s just on a pension how the hell they manage I do not know…having to travel there [hospital] everyday’.(Male, 69)
‘Wherever you go, and you are driving back, you think, “I live here. It’s wonderful.” When you go with the walking group, they all say they are lucky living here. It is completely different here. It is very unthreatening…It gives you an enormous feeling of wellbeing; being here. Just being in this town is a very satisfying place; everybody says hello and smiles. Everybody in this close here knows each other and is friendly. We all have a meal together at Christmas in town; and we are all different ages’.(Female, 68)
3.2.3. Personal Factors
3.2.4. Environmental Factors
’I think the other psychological effect at the time was the journey to and from [hospital] 80, 90 miles. And the appointments were never consistent, you know I could have a late afternoon one and be there for eight o’clock the next morning. That was not great’.(Male, 62)
3.2.5. Specific Healthcare Factors
‘It is funny after you have had all the treatment, and then all of a sudden it’s “See you in a years time”…but you do feel like all of a sudden you have had so much care, and then abandoned’.(Female, 65)
4. Discussion
4.1. Key Findings
4.2. Findings in Context of the Academic Literature
4.3. Strengths
4.4. Limitations
4.5. Implications and Suggestions for Future Research
4.6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Research UK. Cancer Incidence Statistics 2020. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence#heading-Four (accessed on 25 June 2022).
- Cancer Research UK. Cancer Survival Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/survival#heading-Zero (accessed on 25 June 2022).
- Department of Health, Macmillan Cancer Support, NHS Improvement. The National Cancer Survivorship Initiative (NCSI) Vision. 2010. Available online: https://www.pennybrohn.org.uk/wp-content/uploads/2016/11/NCSI_2010.pdf (accessed on 25 June 2022).
- Macmillan Cancer Support. Throwing Light on the Consequences of Cancer and Its Treatment. 2013. Available online: https://www.macmillan.org.uk/documents/aboutus/research/researchandevaluationreports/throwinglightontheconsequencesofcanceranditstreatment.pdf (accessed on 25 June 2022).
- Elliott, J.; Fallows, A.; Staetsky, L.; Smith, P.W.F.; Foster, C.L.; Maher, E.J.; Corner, J. The health and well-being of cancer survivors in the UK: Findings from a population-based survey. Br. J. Cancer 2011, 105, S11–S20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margariti, C.; Gannon, K.N.; Walsh, J.J.; Green, J.S.A. GP experience and understandings of providing follow-up care in prostate cancer survivors in England. Health Soc. Care Community 2020, 28, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Giudice, M.E.D.; Grunfeld, E.; Harvey, B.J.; Piliotis, E.; Verma, S. Primary Care Physicians’ Views of Routine Follow-Up Care of Cancer Survivors. J. Clin. Oncol. 2009, 27, 3338–3345. [Google Scholar] [CrossRef]
- Watson, E.K.; Rose, P.W.; Loftus, R.; Devane, C. Cancer survivorship: The impact on primary care. Br. J. Gen. Pract. 2011, 61, e763. [Google Scholar] [CrossRef] [Green Version]
- Fitch, M.I.; Lockwood, G.; Nicoll, I. Physical, emotional, and practical concerns, help-seeking and unmet needs of rural and urban dwelling adult cancer survivors. Eur. J. Oncol. Nurs. 2021, 53, 101976. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, D.; Melendez, M.; Kwon, M.; Lathan, C. Access to Cancer Care Resources in a Federally Qualified Health Center: A Mixed Methods Study to Increase the Understanding of Met and Unmet Needs of Cancer Survivors. J. Cancer Educ. 2021, 36, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, K.; Magnus, E.; Lundgren, S.; Reidunsdatter, R.J. Everyday life in breast cancer survivors experiencing challenges: A qualitative study. Scand. J. Occup. Ther. 2018, 25, 298–307. [Google Scholar] [CrossRef]
- Armes, J.; Crowe, M.; Colbourne, L.; Morgan, H.; Murrells, T.; Oakley, C.; Palmer, N.; Ream, E.; Young, A.; Richardson, A. Patients’ Supportive Care Needs Beyond the End of Cancer Treatment: A Prospective, Longitudinal Survey. J. Clin. Oncol. 2009, 27, 6172–6179. [Google Scholar] [CrossRef]
- Foster, C.; Fenlon, D. Recovery and self-management support following primary cancer treatment. Br. J. Cancer 2011, 105, S21–S28. [Google Scholar] [CrossRef] [Green Version]
- Defra Rural Statistics. The 2011 Rural-Urban Classification for Output Areas in England; Department for Environment Food & Rural Affairs: London, UK, 2017. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1009128/RUCOA_leaflet_Jan2017.pdf (accessed on 25 June 2022).
- Carriere, R.; Adam, R.; Fielding, S.; Barlas, R.; Ong, Y.; Murchie, P. Rural dwellers are less likely to survive cancer—An international review and meta-analysis. Health Place 2018, 53, 219–227. [Google Scholar] [CrossRef]
- Jong, K.E.; Smith, D.P.; Yu, X.Q.; O’Connell, D.L.; Goldstein, D.; Armstrong, B.K. Remoteness of residence and survival from cancer in New South Wales. Med. J. Aust. 2004, 180, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Afshar, N.; English, D.R.; Milne, R.L. Rural–urban residence and cancer survival in high-income countries: A systematic review. Cancer 2019, 125, 2172–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Kruk, S.R.; Butow, P.; Mesters, I.; Boyle, T.; Olver, I.; White, K.; Sabesan, S.; Zielinski, R.; Chan, B.A.; Spronk, K.; et al. Psychosocial well-being and supportive care needs of cancer patients and survivors living in rural or regional areas: A systematic review from 2010 to 2021. Supportive Care Cancer 2022, 30, 1021–1064. [Google Scholar] [CrossRef] [PubMed]
- Pascal, J.; Johnson, N.; Dickson-Swift, V.; Kenny, A. Returning home: Psychosocial care during the re-entry phase of cancer survivorship in rural Australia. Eur. J. Cancer Care 2015, 24, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Reid-Arndt, S.A.; Cox, C.R. Does Rurality Affect Quality of Life Following Treatment for Breast Cancer? J. Rural Health 2010, 26, 402–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- East Midlands Cancer Alliance. About Us: East Midlands Cancer Alliance. Available online: https://www.eastmidlandscanceralliance.nhs.uk/about-us (accessed on 25 June 2022).
- Chittem, M. Understanding coping with cancer: How can qualitative research help? J. Cancer Res. Ther. 2014, 10, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Maly, R.C. Qualitative research for the study of cancer and age. Hematol./Oncol. Clin. N. Am. 2000, 14, 79–88. [Google Scholar] [CrossRef]
- Ritchie, J.; Lewis, J.; Nicholls, C.M.; Ormston, R. Qualitative Research Practice: A Guide for Social Science Students and Researchers; Sage: London, UK, 2013. [Google Scholar]
- Pope, C.; Mays, N. Qualitative Research in Health Care; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Weaver, K.E.; Palmer, N.; Lu, L.; Case, L.D.; Geiger, A.M. Rural–urban differences in health behaviors and implications for health status among US cancer survivors. Cancer Causes Control 2013, 24, 1481–1490. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Payne, J.B.; Dance, K.V.; Imbody, C.B.; Ho, C.D.; Ayers, A.A.; Flowers, C.R. Priorities for Rural Lymphoma Survivors: A Qualitative Study. Clin. Lymphoma Myeloma Leuk 2020, 20, 47–52.e3. [Google Scholar] [CrossRef] [Green Version]
- McNulty, J.A.; Nail, L. Cancer Survivorship in Rural and Urban Adults: A Descriptive and Mixed Methods Study. J. Rural Health 2015, 31, 282–291. [Google Scholar] [CrossRef]
- Palmer, N.R.; Avis, N.E.; Fino, N.F.; Tooze, J.A.; Weaver, K.E. Rural cancer survivors’ health information needs post-treatment. Patient Educ. Couns. 2020, 103, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Asthana, S.; Gibson, A.; Moon, G.; Brigham, P. Allocating resources for health and social care: The significance of rurality. Health Soc. Care Community 2003, 11, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; McGonagle, I.; Jackson, C.; Kane, R. What is known about the role of rural-urban residency in relation to self-management in people affected by cancer who have completed primary treatment? A scoping review. Supportive Care Cancer 2021, 29, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.E.; Geiger, A.M.; Lu, L.; Case, L.D. Rural-urban disparities in health status among US cancer survivors. Cancer 2013, 119, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunn, K.M.; Berry, N.M.; Meng, X.; Wilson, C.J.; Dollman, J.; Woodman, R.J.; Clark, R.A.; Koczwara, B. Differences in the health, mental health and health-promoting behaviours of rural versus urban cancer survivors in Australia. Supportive Care Cancer 2020, 28, 633–643. [Google Scholar] [CrossRef]
- Pascal, J.; Johnson, N.; Dickson-Swift, V.; McGrath, P.; Dangerfield, F. Understanding receptivity to informal supportive cancer care in regional and rural Australia: A Heideggerian analysis. Eur. J. Cancer Care 2016, 25, 381–390. [Google Scholar] [CrossRef]
- Nelson, D.; Law, G.R.; McGonagle, I.; Turner, P.; Jackson, C.; Kane, R. The Effect of Rural Residence on Cancer-Related Self-Efficacy with UK Cancer Survivors Following Treatment. J. Rural Health 2022, 38, 28–33. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D. A Rural-Urban Comparison of Self-Management in People Affected by Cancer Following Treatment: A Mixed Methods Study. PhD. Thesis, University of Lincoln, Lincoln, UK, 2020. [Google Scholar]
- Corbin, J.; Morse, J.M. The Unstructured Interactive Interview: Issues of Reciprocity and Risks when Dealing with Sensitive Topics. Qual. Inq. 2003, 9, 335–354. [Google Scholar] [CrossRef]
- Gale, N.K.; Heath, G.; Cameron, E.; Rashid, S.; Redwood, S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Bibby, P. The 2011 Rural-Urban Classification for Small Area Geographies: A User Guide and Frequently Asked Questions *v1.0). 2013. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/239478/RUC11user_guide_28_Aug.pdf (accessed on 25 June 2022).
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef] [Green Version]
- Howitt, D. Introduction to Qualitative Methods in Psychology; Pearson Education Ltd.: Harlow, UK, 2010. [Google Scholar]
- Attride-Stirling, J. Thematic networks: An analytic tool for qualitative research. Qual. Res. 2001, 1, 385–405. [Google Scholar] [CrossRef]
- Pasch, J.A.; MacDermid, E.; Velovski, S. Effect of rurality and socioeconomic deprivation on presentation stage and long-term outcomes in patients undergoing surgery for colorectal cancer. ANZ J. Surg. 2021, 91, 1569–1574. [Google Scholar] [CrossRef]
- Van Leeuwen, M.; Husson, O.; Alberti, P.; Arraras, J.I.; Chinot, O.L.; Costantini, A.; Darlington, A.-S.; Dirven, L.; Eichler, M.; Hammerlid, E.B.; et al. Understanding the quality of life (QOL) issues in survivors of cancer: Towards the development of an EORTC QOL cancer survivorship questionnaire. Health Qual. Life Outcomes 2018, 16, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, J.D.; Walter, F.M.; Gray, V.; Sinclair, C.; Howting, D.; Bulsara, M.; Bulsara, C.; Webster, A.; Auret, K.; Saunders, C.; et al. Diagnosing cancer in the bush: A mixed-methods study of symptom appraisal and help-seeking behaviour in people with cancer from rural Western Australia. Fam. Pract. 2013, 30, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goins, R.T.; Williams, K.A.; Carter, M.W.; Spencer, S.M.; Solovieva, T. Perceived Barriers to Health Care Access among Rural Older Adults: A Qualitative Study. J. Rural Health 2005, 21, 206–213. [Google Scholar] [CrossRef]
- Silverman, D. Doing Qualitative Research, 4th ed.; Sage: London, UK, 2017. [Google Scholar]
- Charmaz, K. Stories of Suffering: Subjective Tales and Research Narratives. Qual. Health Res. 1999, 9, 362–382. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Successful Qualitative Research: A Practical Guide for Beginners; Sage: London, UK, 2013. [Google Scholar]
- Braun, V.; Clarke, V. Thematic Analysis: The University of Auckland; 2019. Available online: https://www.psych.auckland.ac.nz/en/about/thematic-analysis.html (accessed on 25 June 2022).
- Muula, A. How do we define ‘rurality’ in the teaching on medical demography? Rural Remote Health 2007, 7, 653. [Google Scholar] [CrossRef]
- Nelson, K.S.; Nguyen, T.D.; Brownstein, N.A.; Garcia, D.; Walker, H.C.; Watson, J.T.; Xin, A. Definitions, measures, and uses of rurality: A systematic review of the empirical and quantitative literature. J. Rural Stud. 2021, 82, 351–365. [Google Scholar] [CrossRef]
| Inclusion | Exclusion |
|---|---|
| ≥18 years old | <18 years old |
| Diagnosis of cancer and registration in the database of cancer patients at largest acute NHS Trusts at the study sites | Metastatic spread and/or evidential cancer recurrence |
| Have undergone primary cancer treatment up to 5 years ago | Cancer treatment initiated within the last year |
| Willing and able to give informed consent | Currently under palliative care |
| Adequate level of spoken and written English | Spoken and written English not at adequate level |
| All Respondents (N = 16) | n (%) | |
|---|---|---|
| Age (at start of study) | 35–44 | 1 (6.3) |
| 45–54 | 1 (6.3) | |
| 55–64 | 7 (43.7) | |
| 65–74 | 5 (31.2) | |
| Over 75 | 2 (12.5) | |
| Gender | Female | 10 (62.5) |
| Male | 6 (37.5) | |
| Living arrangement | Partner/spouse/family/friends | 13 (81.2) |
| Alone | 2 (12.5) | |
| Missing data | 1 (6.3) | |
| Marital status | Married/civil partnership | 12 (75.0) |
| Living with partner | 1 (6.3) | |
| Widowed | 2 (12.4) | |
| Missing data | 1 (6.3) | |
| Employment status | Employed | 3 (18.7) |
| Retired | 10 (62.5) | |
| Other | 2 (12.5) | |
| Missing data | 1 (6.3) | |
| Annual household income | £0–14,999 | 2 (12.5) |
| £15–24,999 | 3 (18.8) | |
| £25–49,999 | 5 (31.1) | |
| £50–74,999 | 1 (6.3) | |
| Over £75,000 | 2 (12.5) | |
| Missing data | 3 (18.8) | |
| Residence—degree of rurality | Rural town and fringe | 6 (37.5) |
| Rural village | 6 (37.5) | |
| Rural village in sparse setting | 1 (6.3) | |
| Rural hamlet and isolated dwelling | 3 (18.7) | |
| Primary cancer type | Breast | 4 (25.0) |
| Urological | 3 (18.7) | |
| Skin | 2 (12.5) | |
| Head and neck | 3 (18.7) | |
| Gynaecological | 2 (12.5) | |
| Lower gastrointestinal | 1 (6.3) | |
| Haematological | 1 (6.3) |
| Category | Subcategory | Explanation | Example |
|---|---|---|---|
| Problematic events | Cancer diagnosis | The process of a diagnosis of cancer | ‘I had gone in and never thought for one minute that I had got cancer…I got taken into the room with a consultant and the Macmillan nurse. And she just said straight away, “Well, I’m sorry, you have got cancer.” Well…you think the worst don’t you…that was just a huge shock…cancer had never been discussed.’ (Female, 57) |
| Cancer treatment | Any part of the treatment process | ‘So I had surgery and twenty three lymph nodes removed; of which eighteen were fine. The others were showing signs of having cancer. It was awful.’ (Female, 65) | |
| Cancer recovery | Any issues or obstacles following treatment | ‘I hadn’t appreciated quite how long the side effects, in terms of tiredness and energy levels were going to continue for.’ (Female, 39) | |
| Fear of recurrence | The worry that cancer will return | ‘You get really obsessed about it coming back. Or every ache and pain—“Is that cancer!”’ (Female, 39) | |
| Pre-existing factors | Age | ‘One of those things that most people my age when they get to their 60s and 70s they have got these cells in their bloodstream and most people live with it harmlessly.’ (Male, 69) | |
| Gender | ‘Support for men, is pretty dire, pretty dire.’ (Male, 62) | ||
| Marital status | Partnership or lack thereof, divorce, widow/er | ‘I am married and have been for 40 years.’ (Female, 62) | |
| Living arrangement | Who the participant lives with | ‘My house is too big for me; but I’ll stick here until they carry me out.’ (Female, 81) | |
| Socioeconomic status | Household income, employment status, housing, resources available to the participant | ‘I am just fortunate in my lifestyle um [pause] which means I don’t have to have those added pressures you know I don’t have to earn money because I have done that in my earlier life which is now set me up for my later life.’ (Female, 55) | |
| Comorbidities | Any other conditions the participant has | ‘I’ve got labrynthitis, occasionally my ear becomes infected and I get sick and fall over.’ (Female, 68) | |
| Familial support | Available help from family members | ‘My mum in law used to come a lot with me so I could remember everything that we discussed.’ (Female, 39) | |
| Employment | Whether or not the participant is in work and how they perceive it | ‘Being freelance there is no “If I don’t work”; you know you don’t work you don’t earn sort of thing.’ (Male, 53) | |
| Rurality | Setting they live in | ‘Very flat, very rural, largely agricultural. Little in the way of large settlement. This village is very quiet indeed.’ (Male, 62) | |
| Personal factors | Interpretations of life/self-conduct | How the participant views life and how they carry themselves | ‘I think when you get older, you get things in perspective a little easier, than when you were younger.’ (Female, 58) |
| Identity | How the participant views themselves, the activities they enjoy, and their personality | ‘I’ve always been an organiser, that has kept me going and I do a lot of organising.’ (Female, 68) | |
| Stoicism | Continuing through adversity in life with no complaint or outwardly viewable suffering | ‘I’ve survived on my own and coped quite well.’ (Female, 81) | |
| Attitude to health | How the participant views their health and looks after it | ‘I just think if your number is up, your number is up.’ (Female, 58) | |
| Self-management | How they look after their own health, behaviour, and lifestyle | ‘Self-management is about I guess, taking ownership of your own body and learning that you can impact it yourself, by taking care of yourself in different ways.’ (Female, 39) | |
| Environmental factors | Travel | Public transportation, travel by car, and distance from amenities | ‘There are no buses down here, you either have to get a car, or walk.’ (Female, 57) |
| Type of rurality | How dispersed the population is | The UK Office for National Statistics (ONS) RUC2011 Rural Urban Classification (e.g., Rural Village in a Sparse Setting) | |
| Community support | Available help from others in the local area and local recreational activities | ‘If anything goes wrong, you know that you can just run down the lane and there will be somebody there that can help you out.’ (Female, 58) | |
| Access to support | How available structural support, such as healthcare professionals and support groups, is to the participants | ‘There is a doctor surgery at the village we are in.’ (Male, 53) | |
| Healthcare resources | Facilities, personnel, and funds for healthcare provision (or lack thereof) | ‘There was a severe shortage of beds.’ (Male, 70) | |
| Specific healthcare factors | Follow-up | Any contact with healthcare staff after treatment | ‘I had six week reviews. They were later moved up to three months.’ (Male, 62) |
| Type of treatment | What sort of intervention the participant had for their cancer | ‘Then I went for chemo, radiotherapy for 28 days.’ (Female, 62) | |
| Type of cancer | The specific diagnosis | ‘Stage 2 bowel cancer.’ (Female, 62) | |
| Relationship with healthcare professionals | The trust, belief, and opinions that the participants have in clinicians | ‘There has been the occasional person I have not been happy with, but on the whole for my cancer treatment, they have been very good.’ (Female, 68) | |
| Healthcare information needs | Any need felt by the participants for more knowledge of their condition, treatment, or health status | ‘There isn’t really that much information about how to manage yourself going forward.’ (Female, 39) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graham, F.; Kane, R.; Gussy, M.; Nelson, D. Recovery of Health and Wellbeing in Rural Cancer Survivors Following Primary Treatment: Analysis of UK Qualitative Interview Data. Nurs. Rep. 2022, 12, 482-497. https://doi.org/10.3390/nursrep12030046
Graham F, Kane R, Gussy M, Nelson D. Recovery of Health and Wellbeing in Rural Cancer Survivors Following Primary Treatment: Analysis of UK Qualitative Interview Data. Nursing Reports. 2022; 12(3):482-497. https://doi.org/10.3390/nursrep12030046
Chicago/Turabian StyleGraham, Florence, Ros Kane, Mark Gussy, and David Nelson. 2022. "Recovery of Health and Wellbeing in Rural Cancer Survivors Following Primary Treatment: Analysis of UK Qualitative Interview Data" Nursing Reports 12, no. 3: 482-497. https://doi.org/10.3390/nursrep12030046
APA StyleGraham, F., Kane, R., Gussy, M., & Nelson, D. (2022). Recovery of Health and Wellbeing in Rural Cancer Survivors Following Primary Treatment: Analysis of UK Qualitative Interview Data. Nursing Reports, 12(3), 482-497. https://doi.org/10.3390/nursrep12030046







