The Interplay Between β-Thalassemia and the Human Virome: Immune Dysregulation, Viral Reactivation, and Clinical Implications
Abstract
1. Introduction
2. Methods for Literature Review
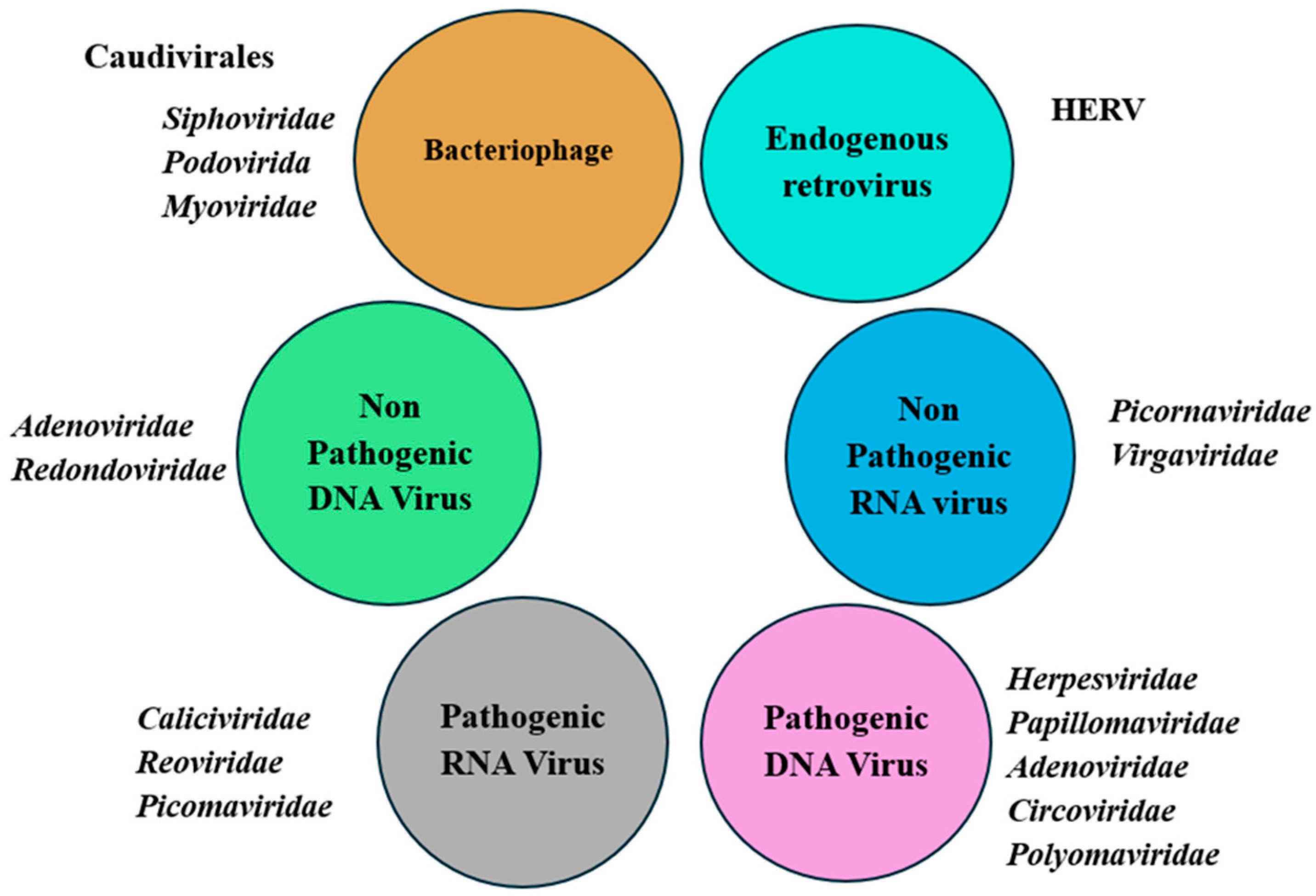
3. Composition of Human Virome
4. Neutrophil-Associated Immune Dysregulation in β-Thalassemia: Implications for Human Virome Alterations
5. Lymphocytes (T Cell, B Cell, NK Cells)-Associated Immune Dysregulation in β-Thalassemia: Implications for Human Virome Alterations
6. Iron Overload and Increased Virome Susceptibility in β-Thalassemia
7. Transfusion and Increased Virome Susceptibility in β-Thalassemia Patients
8. Possible Role of Bacteriophages in Gut Dysbiosis and Immune Modulation in Transfusion-Dependent β-Thalassemia Patients
9. Allogeneic Hematopoietic Stem Cell Transplantation and Reactivation Risk of Virus in β-Thalassemia Patients
10. Risk of Virus Reactivation After Splenectomy in β-Thalassemia Patients
11. Conclusions: Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| β-TM | Beta-thalassemia major |
| TI | Beta-thalassemia intermedia |
| TDT | Transfusion-dependent thalassemia |
| NTDT | Non-transfusion-dependent thalassemia |
| TTIs | Transfusion-transmitted infections |
| allo-HSCT | Allogeneic hematopoietic stem cell transplantation |
| CMV | Cytomegalovirus |
| HTLV-I/II | Human T-cell lymphotropic virus types I and II |
| MDSCs | Myeloid-derived suppressor cells |
| PTX3 | Pentraxin 3 |
| CXCR2/CXCL2 | CXC chemokine receptor 2/chemokine ligand 2 |
| LPS | Lipopolysaccharide |
| aGvHD | Acute graft-versus-host disease |
| VZV | Varicella-zoster virus |
References
- Iyevhobu, K.O.; Okobi, T.J.; Usoro, E.R.; Ivie, A.A.; Ken-Iyevhobu, B.A.; Victoria, O.O. Overview of beta-thalassemia. In Thalassemia Syndromes—New Insights and Transfusion Modalities; IntechOpen: London, UK, 2023. [Google Scholar]
- Basu, S.; Rahaman, M.; Dolai, T.K.; Shukla, P.C.; Chakravorty, N. Understanding the intricacies of iron overload associated with β-thalassemia: A comprehensive review. Thalass. Rep. 2023, 13, 179–194. [Google Scholar] [CrossRef]
- Ferdous, J.; Tasnim, M.; Qadri, F.; Hosen, M.I.; Chowdhury, E.K.; Shekhar, H.U. Disease-Modifying Effect of HBS1L-MYB in HbE/β-Thalassemia Patients in Bangladeshi Population. Thalass. Rep. 2024, 14, 103–117. [Google Scholar] [CrossRef]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Thein, S.L. Pathophysiology of β thalassemia—A guide to molecular therapies. ASH Educ. Program Book 2005, 2005, 31–37. [Google Scholar] [CrossRef]
- Bou-Fakhredin, R.; De Franceschi, L.; Motta, I.; Cappellini, M.D.; Taher, A.T. Pharmacological induction of fetal hemoglobin in β-thalassemia and sickle cell disease: An updated perspective. Pharmaceuticals 2022, 15, 753. [Google Scholar] [CrossRef]
- Rivella, S. β-thalassemias: Paradigmatic diseases for scientific discoveries and development of innovative therapies. Haematologica 2015, 100, 418. [Google Scholar] [CrossRef]
- Bai, G.H.; Lin, S.C.; Hsu, Y.H.; Chen, S.Y. The human virome: Viral metagenomics, relations with human diseases, and therapeutic applications. Viruses 2022, 14, 278. [Google Scholar] [CrossRef]
- Coskun, O.; Sener, K.; Kilic, S.; Erdem, H.; Yaman, H.; Besirbellioglu, A.B.; Gul, H.C.; Eyigun, C.P. Stress-related Epstein–Barr virus reactivation. Clin. Exp. Med. 2010, 10, 15–20. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Rodriguez, T.M.; Hollister, E.B. Human virome and disease: High-throughput sequencing for virus discovery, identification of phage-bacteria dysbiosis and development of therapeutic approaches with emphasis on the human gut. Viruses 2019, 11, 656. [Google Scholar] [CrossRef]
- Zárate, S.; Taboada, B.; Yocupicio-Monroy, M.; Arias, C.F. Human virome. Arch. Med. Res. 2017, 48, 701–716. [Google Scholar] [CrossRef]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef]
- Siwaponanan, P.; Siegers, J.Y.; Ghazali, R.; Ng, T.; McColl, B.; Ng, G.Z.W.; Sutton, P.; Wang, N.; Ooi, I.; Thiengtavor, C.; et al. Reduced PU.1 expression underlies aberrant neutrophil maturation and function in β-thalassemia mice and patients. Blood J. Am. Soc. Hematol. 2017, 129, 3087–3099. [Google Scholar] [CrossRef]
- Kabir, T.; Anwar, S.; Mourosi, J.T.; Akter, S.; Hosen, M.J. α-and β-Globin Gene Mutations in Individuals with Hemoglobinopathies in the Chattogram and Sylhet Regions of Bangladesh. Hemoglobin 2023, 47, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, J.; Vahidshahi, K.; Kosaryan, M.; Parvinnejad, N.; Mahdavi, M.; Karami, H. Nitroblue tetrazolium test in patients with beta-thalassemia major. Saudi Med. J. 2008, 29, 1601–1605. [Google Scholar]
- Elsayh, K.I.; Mohammed, W.S.; Zahran, A.M.; Saad, K. Leukocytes apoptosis and adipocytokines in children with beta thalassemia major. Clin. Exp. Med. 2016, 16, 345–350. [Google Scholar] [CrossRef]
- Bazi, A.; Shahramian, I.; Yaghoobi, H.; Naderi, M.; Azizi, H. The role of immune system in thalassemia major: A narrative review. J. Pediatr. Rev. 2018, 6, 29–36. [Google Scholar] [CrossRef]
- Amer, J.; Fibach, E. Chronic oxidative stress reduces the respiratory burst response of neutrophils from beta-thalassaemia patients. Br. J. Haematol. 2005, 129, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.B.; Porter, J.; Evans, P.; Kwiatkowski, J.L.; Neufeld, E.J.; Coates, T.; Giardina, P.J.; Grady, R.W.; Vichinsky, E.; Olivieri, N.; et al. Increased leucocyte apoptosis in transfused β-thalassaemia patients. Br. J. Haematol. 2013, 160, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Dakic, A.; Metcalf, D.; Di Rago, L.; Mifsud, S.; Wu, L.; Nutt, S.L. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J. Exp. Med. 2005, 201, 1487–1502. [Google Scholar] [CrossRef]
- Will, B.; Vogler, T.O.; Narayanagari, S.; Bartholdy, B.; Todorova, T.I.; da Silva Ferreira, M.; Chen, J.; Yu, Y.; Mayer, J.; Barreyro, L.; et al. Minimal PU.1 reduction induces a preleukemic state and promotes development of acute myeloid leukemia. Nat. Med. 2015, 21, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.L. Neutrophils deficient in PU.1 do not terminally differentiate or become functionally competent. Blood J. Am. Soc. Hematol. 1998, 92, 1576–1585. [Google Scholar]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.; Turk, M.; Jenne, C.N.; Kim, S.J. Neutrophils in viral infection. Cell Tissue Res. 2018, 371, 505–516. [Google Scholar] [CrossRef]
- Pentraxin, P.T.X. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J. Immunol. 2008, 180, 3391–3398. [Google Scholar] [CrossRef]
- Johansson, C.; Kirsebom, F.C. Neutrophils in respiratory viral infections. Mucosal Immunol. 2021, 14, 815–827. [Google Scholar] [CrossRef]
- Hazrati, E.; Galen, B.; Lu, W.; Wang, W.; Ouyang, Y.; Keller, M.J.; Lehrer, R.I.; Herold, B.C. Human α-and β-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 2006, 177, 8658–8666. [Google Scholar] [CrossRef]
- Wang, W.; Owen, S.M.; Rudolph, D.L.; Cole, A.M.; Hong, T.; Waring, A.J.; Lal, R.B.; Lehrer, R.I. Activity of α-and θ-defensins against primary isolates of HIV-1. J. Immunol. 2004, 173, 515–520. [Google Scholar] [CrossRef]
- Hayashi, K.; Hooper, L.C.; Okuno, T.; Takada, Y.; Hooks, J.J. Inhibition of HSV-1 by chemoattracted neutrophils: Supernatants of corneal epithelial cells (HCE) and macrophages (THP-1) treated with virus components chemoattract neutrophils (PMN), and supernatants of PMN treated with these conditioned media inhibit viral growth. Arch. Virol. 2012, 157, 1377–1381. [Google Scholar] [CrossRef]
- Klebanoff, S.J.; Coombs, R.W. Viricidal effect of polymorphonuclear leukocytes on human immunodeficiency virus-1. Role of the myeloperoxidase system. J. Clin. Investig. 1992, 89, 2014–2017. [Google Scholar] [CrossRef]
- Skulachev, V. Possible role of reactive oxygen species in antiviral defense. BIOCHEM. C/C BIOKHIMIIA 1998, 63, 1438–1440. [Google Scholar]
- Galani, I.E.; Andreakos, E. Neutrophils in viral infections: Current concepts and caveats. J. Leucoc. Biol. 2015, 98, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Sae-Khow, K.; Charoensappakit, A.; Leelahavanichkul, A. Neutrophil diversity (immature, aged, and low-density neutrophils) and functional plasticity: Possible impacts of iron overload in β-thalassemia. Int. J. Mol. Sci. 2024, 25, 10651. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Kong, K.F.; Dai, J.; Qian, F.; Zhang, L.; Brown, C.R.; Fikrig, E.; Montgometry, R.R. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J. Infect. Dis. 2010, 202, 1804–1812. [Google Scholar] [CrossRef]
- Dejucq, N.; Jégou, B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol. Mol. Biol. Rev. 2001, 65, 208–231. [Google Scholar] [CrossRef]
- Noulsri, E.; Lerdwana, S.; Fucharoen, S.; Pattanapanyasat, K. Phenotypic characterization of circulating CD4/CD8 T-lymphocytes in β-thalassemia patients. Asian Pac. J. Allergy Immunol. 2014, 32, 261–269. [Google Scholar]
- Pourgheysari, B.; Karimi, L.; Beshkar, P. Alteration of T cell subtypes in beta-thalassaemia major: Impact of ferritin level. J. Clin. Diagn. Research JCDR 2016, 10, DC14. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Fulbright, J.W.; Crowson, C.S.; Poland, G.A.; O’Fallon, W.M.; Weyand, C.M. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 2001, 75, 12182–12187. [Google Scholar] [CrossRef]
- Carmona-Rivera, C.; Kaplan, M.J. Low-density granulocytes: A distinct class of neutrophils in systemic autoimmunity. Semin. Immunopathol. 2013, 35, 455–463. [Google Scholar] [CrossRef]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 2019, 25, 285–299. [Google Scholar] [CrossRef]
- Schoggins, J.W. Recent advances in antiviral interferon-stimulated gene biology. F1000Research 2018, 7, 309. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.; Han, O. Systemic iron status. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2009, 1790, 584–588. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron metabolism in ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Malcovati, L. Impact of transfusion dependency and secondary iron overload on the survival of patients with myelodysplastic syndromes. Leuk. Res. 2007, 31, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Melchiori, L.; Gardenghi, S.; Rivella, S. β-thalassemia: hiJAKing ineffective erythropoiesis and iron overload. Adv. Hematol. 2010, 2010, 938640. [Google Scholar] [CrossRef]
- Gardenghi, S.; Grady, R.W.; Rivella, S. Anemia, ineffective erythropoiesis and hepcidin: Interacting factors in abnormal iron metabolism leading to iron overload in β-thalassemia. Hematol./Oncol. Clin. North Am. 2010, 24, 1089. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552. [Google Scholar] [CrossRef]
- Wei, Y.; Ye, W.; Zhao, W. Serum iron levels decreased in patients with HBV-related hepatocellular carcinoma, as a risk factor for the prognosis of HBV-related HCC. Front. Physiol. 2018, 9, 66. [Google Scholar] [CrossRef]
- Di Bisceglie, A.M.; Axiotis, C.A.; Hoofnagle, J.H.; Bacon, B.R. Measurements of iron status in patients with chronic hepatitis. Gastroenterology 1992, 102, 2108–2113. [Google Scholar] [CrossRef]
- Xu, M.; Kashanchi, F.; Foster, A.; Rotimi, J.; Turner, W.; Gordeuk, V.R.; Nekhai, S. Hepcidin induces HIV-1 transcription inhibited by ferroportin. Retrovirology 2010, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Nekhai, S.; Kumari, N.; Dhawan, S. Role of cellular iron and oxygen in the regulation of HIV-1 infection. Future Virol. 2013, 8, 301–311. [Google Scholar] [CrossRef]
- Mancone, C.; Grimaldi, A.; Refolo, G.; Abbate, I.; Rozera, G.; Benelli, D.; Fimia, G.M.; Barnaba, V.; Tripodi, M.; Piacentini, M.; et al. Iron overload down-regulates the expression of the HIV-1 Rev cofactor eIF5A in infected T lymphocytes. Proteome Sci. 2017, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Vento, S.; Cainelli, F.; Cesario, F. Infections and thalassaemia. Lancet Infect. Dis. 2006, 6, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Xie, C.; Kirkness, E.; Biggs, W.; Wong, E.; Turpaz, Y.; Bloom, K.; Delwart, E.; Nelson, K.E.; Venter, J.C.; et al. The blood DNA virome in 8000 humans. PLoS Pathog. 2017, 13, e1006292. [Google Scholar]
- Zurlo, M.; De Stefano, P.; Borgna-Pignatti, C.; Di Palma, A.; Melevendi, C.; Piga, A.; Di Gregorio, F.; Burattini, M.; Terzoli, S. Survival and causes of death in thalassaemia major. Lancet 1989, 334, 27–30. [Google Scholar] [CrossRef]
- Abdelmawla, D.; Moemen, D.; Darwish, A.; Mowafy, W. Hepatitis E virus prevalence in Egyptian children with transfusion-dependent thalassemia. Braz. J. Infect. Dis. 2019, 23, 40–44. [Google Scholar] [CrossRef]
- Farshadpour, F.; Taherkhani, R.; Shaeri, M. Prevalence and risk factors of hepatitis E virus infection among patients with β-thalassemia major in South of Iran. J. Immunoass. Immunochem. 2022, 43, 452–462. [Google Scholar] [CrossRef]
- Al-Fawaz, I.; Al-Rasheed, S.; Al-Mugeiren, M.; Al-Salloum, A.; Al-Sohaibani, M.; Ramia, S. Hepatitis E virus infection in patients from Saudi Arabia with sickle cell anaemia and β-thalassemia major: Possible transmission by blood transfusion. J. Viral Hepat. 1996, 3, 203–205. [Google Scholar] [CrossRef]
- Slavov, S.N.; Maçonetto, J.D.; Martinez, E.Z.; Silva-Pinto, A.C.; Covas, D.T.; Eis-Hübinger, A.M.; Kashima, S. Prevalence of hepatitis E virus infection in multiple transfused Brazilian patients with thalassemia and sickle cell disease. J. Med. Virol. 2019, 91, 1693–1697. [Google Scholar] [CrossRef]
- Nigam, N.; Kushwaha, R.; Yadav, G.; Singh, P.K.; Gupta, N.; Singh, B.; Agrawal, M.; Chand, P.; Saxena, S.K.; Bhatt, M.L.B. A demographic prevalence of β Thalassemia carrier and other hemoglobinopathies in adolescent of Tharu population. J. Fam. Med. Prim. Care 2020, 9, 4305–4310. [Google Scholar] [CrossRef]
- Prati, D. Benefits and complications of regular blood transfusion in patients with beta-thalassaemia major. Vox Sang. 2000, 79, 129–137. [Google Scholar] [CrossRef]
- Chalabi, D.A.; Al-Azzawi, S. Antiviral Treatment of Chronic Hepatitis C Infection among Children and Adolescents with Beta-Thalassemia Major. Med. J. Babylon 2019, 16, 340–345. [Google Scholar] [CrossRef]
- Gorski, A.; Międzybrodzki, R.; Jończyk-Matysiak, E.; Żaczek, M.; Borysowski, J. Phage-specific diverse effects of bacterial viruses on the immune system. Future Microbiol. 2019, 14, 1171–1174. [Google Scholar] [CrossRef]
- Penadés, J.R.; Chen, J.; Quiles-Puchalt, N.; Carpena, N.; Novick, R.P. Bacteriophage-mediated spread of bacterial virulence genes. Curr. Opin. Microbiol. 2015, 23, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sweere, J.M.; Van Belleghem, J.D.; Ishak, H.; Bach, M.S.; Popescu, M.; Sunkari, V.; Kaber, G.; Manasherob, R.; Suh, G.A.; Cao, X.; et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 2019, 363, eaat9691. [Google Scholar] [CrossRef]
- Robinson, C.M.; Jesudhasan, P.R.; Pfeiffer, J.K. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 2014, 15, 36–46. [Google Scholar] [CrossRef]
- Dou, H.H.; Luo, J.M.; Zhao, Y.J.; Wang, J.G.; Qin, Y.H. Risk factors for hemorrhagic cystitis in children with severe beta-thalassemia after allogeneic hematopoietic stem cell transplantation. Front. Pediatr. 2025, 13, 1558099. [Google Scholar] [CrossRef]
- Knöll, A.; Boehm, S.; Hahn, J.; Holler, E.; Jilg, W. Reactivation of resolved hepatitis B virus infection after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2004, 33, 925–929. [Google Scholar] [CrossRef]
- Rafique, N.; Ali, A.; Ghaffor, T.; Khattak, T.A.; Asghar, M.B.; Chaudhry, Q.U.N. Acute Graft versus Host Disease in Beta Thalassemia Patients Following Allogeneic Haematopoietic Stem Cell Transplantation. J. Coll. Physicians Surg.-Pak. JCPSP 2024, 34, 480–483. [Google Scholar] [PubMed]
- Düver, F.; Weißbrich, B.; Eyrich, M.; Wölfl, M.; Schlegel, P.G.; Wiegering, V. Viral reactivations following hematopoietic stem cell transplantation in pediatric patients—A single center 11-year analysis. PLoS ONE 2020, 15, e0228451. [Google Scholar] [CrossRef]
- Ljungman, P.; De La Camara, R.; Cordonnier, C.; Einsele, H.; Engelhard, D.; Reusser, P.; Styczynski, J.; Ward, K. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008, 42, 227–240. [Google Scholar] [CrossRef]
- Kurtoğlu, A.U.; Uğur, S.; Göçer, M.; Kurtoğlu, E. Effects of Splenectomy on Natural Killer Cell Levels in β-Thalassemia Major Patients. J. Clin. Lab. Anal. 2024, 38, e25046. [Google Scholar] [CrossRef]
- Akca, T.; Ozdemir, G.N.; Aycicek, A.; Ozkaya, G. Long-term results of splenectomy in transfusion-dependent thalassemia. J. Pediatr. Hematol./Oncol. 2023, 45, 143–148. [Google Scholar] [CrossRef]
- Borgers, J.S.; Tobin, R.P.; Vorwald, V.M.; Smith, J.M.; Davis, D.M.; Kimball, A.K.; Clambey, E.T.; Couts, K.L.; McWilliams, J.A.; Jordan, K.R.; et al. High-dimensional analysis of postsplenectomy peripheral immune cell changes. Immunohorizons 2020, 4, 82–92. [Google Scholar] [CrossRef]
- Becker, P.S.; Suck, G.; Nowakowska, P.; Ullrich, E.; Seifried, E.; Bader, P.; Tonn, T.; Seidl, C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016, 65, 477–484. [Google Scholar] [CrossRef]
- Arslan, B.A.; Erdem-Kuruca, S.; Karakas, Z.; Erman, B.; Ergen, A. Effects of micro environmental factors on natural killer activity (NK) of beta thalassemia major patients. Cell. Immunol. 2013, 282, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gluba-Brzózka, A.; Franczyk, B.; Rysz-Górzyńska, M.; Rokicki, R.; Koziarska-Rościszewska, M.; Rysz, J. Pathomechanisms of immunological disturbances in β-thalassemia. Int. J. Mol. Sci. 2021, 22, 9677. [Google Scholar] [CrossRef] [PubMed]
- Ammar, S.A.; Elsayh, K.I.; Zahran, A.M.; Embaby, M. Splenectomy for patients with β-thalassemia major: Long-term outcomes. Egypt. J. Surg. 2014, 33, 232–236. [Google Scholar] [CrossRef]


| Different Body Part | Virome Group | Example | Phage Group | Example |
|---|---|---|---|---|
| Central Nervous System | Herpesviruses | HSV-1, HSV-2 | Siphoviridae | λ phage, Streptococcus phage φC1 |
| Podoviridae | T7 phage, Bacillus phage φ29 | |||
| Polyomaviruses | JC virus, BK virus | |||
| Myoviridae | T4 phage, Pseudomonas phage PB1 | |||
| Eyes | Herpesviruses | HSV-1 | Siphoviridae | λ phage, Streptococcus phage φC1 |
| Adenoviruses | Adenovirus serotype 8, | Podoviridae | T4 phage, Pseudomonas phage PB1 | |
| Myoviridae | λ phage, Streptococcus phage φC1 | |||
| Papillomaviruses | HIV-16 | |||
| Microviridae | φX174 | |||
| Oral/Nasal Cavity | Anelloviruses | Torque teno virus (TTV), | Siphoviridae | λ phage, Streptococcus phage φC1 |
| Herpesviruses | EBV, HSV-1 | Myoviridae | T4 phage, Pseudomonas phage PB1 | |
| Papillomaviruses | HPV | Leviviridae | MS2 | |
| Lungs | Anelloviruses | TTV | Podoviridae | T7 phage, Bacillus phage φ29 |
| Paramyxoviruses | RSV, HMPV | |||
| Myoviridae | T4 phage, Pseudomonas phage PB1 | |||
| Influenza viruses | Influenza A | |||
| Gastrointestinal Tract | Enteric viruses | Norovirus, Rotavirus, Astrovirus | Siphoviridae | λ phage, Streptococcus phage φC1 |
| Podoviridae | T7 phage, Bacillus phage φ29 | |||
| Myoviridae | T4 phage, Pseudomonas phage PB1 | |||
| Anelloviruses | TTV | |||
| Microviridae | φX174 | |||
| Inoviridae | M13 phage | |||
| Adenoviruses | Adenovirus 41 | |||
| Leviviridae | MS2 | |||
| Skin | Papillomaviruses | HPV-1, HPV-8 | Siphoviridae | λ phage, Streptococcus phage φC1 |
| Myoviridae | T4 phage, Pseudomonas phage PB1 | |||
| Polyomaviruses | Merkel cell polyomavirus, TTV | |||
| Inoviridae | M13 phage | |||
| Blood (Plasma) | Anelloviruses | TTV | Siphoviridae | λ phage, Streptococcus phage φC1 |
| Podoviridae | T7 phage, Bacillus phage φ29 | |||
| Herpesviruses | CMV, EBV | Myoviridae | T4 phage, Pseudomonas phage PB1 | |
| Retroviruses | HIV | Microviridae | φX174 | |
| Polyomaviruses | BK virus | Inoviridae | M13 phage | |
| Genitourinary Tract | Herpesviruses | HSV-2, CMV | Siphoviridae | λ phage, Streptococcus phage φC1 |
| Papillomaviruses | HPV-16, HPV-18 | Podoviridae | T7 phage, Bacillus phage φ29 | |
| Polyomaviruses | JC virus | Microviridae | φX174 | |
| Urine | Polyomaviruses | BK virus, JC virus | Siphoviridae | λ phage, Streptococcus phage φC1 |
| Podoviridae | T7 phage, Bacillus phage φ29 | |||
| Anelloviruses | TTV | |||
| Myoviridae | T4 phage, Pseudomonas phage PB1 |
| Patho Mechanism | Description | Affect | Changes in Virome | Ref. |
|---|---|---|---|---|
| Immune dysregulation: Neutrophils | reduction in neutrophil number and function | Viral persistence, reactivation, or opportunistic infection | HIV, hepatitis B and C viruses, rhinovirus, HSV-1, RSV, and influenza | [30] |
| Immune dysregulation: Lymphocytes (T Cell, B Cell, NK Cells) | Senescent CD8+CD28− T, diminished telomerase activity, suppress dendritic cell activation | Influence viral replication, latency, and clearance | HIV, hepatitis B and C viruses, rhinovirus, HSV-1, RSV, and influenza | [30] |
| Iron overload | Suppressing hepcidin expression, a key hormone that regulates systemic iron balance | Excess iron promotes viral infection and progression, Decreased NK activity | HIV, hepatitis B and C viruses | [49] |
| Transfusion | Suboptimal screening of blood donors, inadequate testing, and improper blood processing | Transfusion-transmitted infections(TTIs) | hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), West Nile virus (WNV), and human T-cell lymphotropic viruses I and II (HTLV-I/II). | [54] |
| Bacteriophage dysbiosis | Type 1 IFN inhibits TNF production and limits bacterial phagocytosis | Bacterial lipopolysaccharide enhances virion stability | Poliovirus | [67] |
| Clinical implications: allo-HSTC | various transplantation-related complications | Reactivation | human herpesvirus 6 (HHV-6), Epstein–Barr virus (EBV), cytomegalovirus (CMV), adenovirus (ADV), herpes simplex virus (HSV), and varicella-zoster virus (VZV) | [11] |
| Clinical implications: Splenectomy | Impaired hepcidin–ferroportin response | NK cell number and activity decrease | HIV, hepatitis B and C viruses | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, D.; Hosen, M.J. The Interplay Between β-Thalassemia and the Human Virome: Immune Dysregulation, Viral Reactivation, and Clinical Implications. Thalass. Rep. 2025, 15, 10. https://doi.org/10.3390/thalassrep15040010
Hossain D, Hosen MJ. The Interplay Between β-Thalassemia and the Human Virome: Immune Dysregulation, Viral Reactivation, and Clinical Implications. Thalassemia Reports. 2025; 15(4):10. https://doi.org/10.3390/thalassrep15040010
Chicago/Turabian StyleHossain, Didar, and Mohammad Jakir Hosen. 2025. "The Interplay Between β-Thalassemia and the Human Virome: Immune Dysregulation, Viral Reactivation, and Clinical Implications" Thalassemia Reports 15, no. 4: 10. https://doi.org/10.3390/thalassrep15040010
APA StyleHossain, D., & Hosen, M. J. (2025). The Interplay Between β-Thalassemia and the Human Virome: Immune Dysregulation, Viral Reactivation, and Clinical Implications. Thalassemia Reports, 15(4), 10. https://doi.org/10.3390/thalassrep15040010






