Trigeminal Nerve and Vestibular System: Update on Pathophysiological and Clinical Links
Abstract
1. Introduction
2. Embryogenesis
2.1. Cranial Nerves
2.2. Inner Ear
3. Anatomy
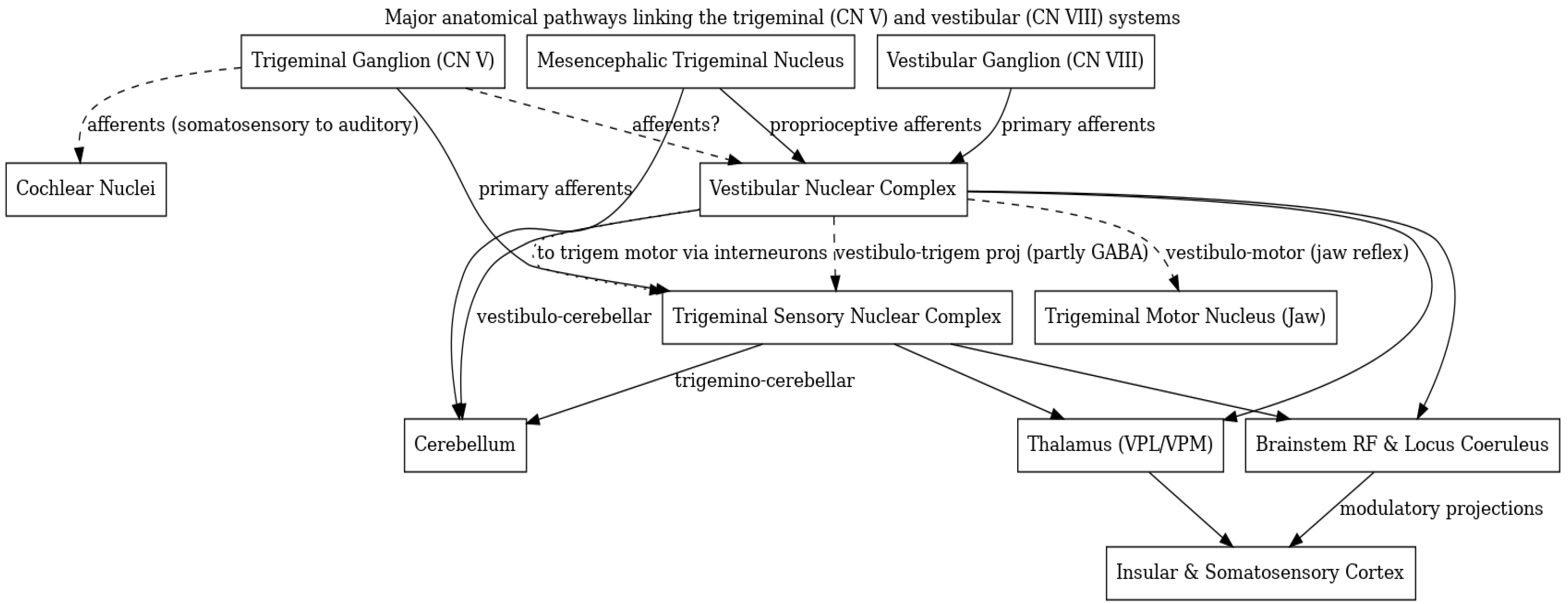
3.1. Peripheral and Cranial Nerve Pathways
3.2. Brainstem and Central Connections
3.3. Trigeminal Afferents to Vestibular Nuclei
3.4. Vestibular Projections to Trigeminal Nuclei
3.5. Common Brainstem Integrative Centers
3.6. Thalamus and Cortex
4. Pathology
4.1. Vestibular Migraine and Ménière’s Disease
- Vascular theory: proposes that transient cerebral hypoxia from vasoconstriction causes aura, followed by reactive vasodilation that triggers headache [56].
- Trigemino-vascular theory
- Cortical spreading depression theory: attributes the migraine to a wave of neuronal depression across the cortex.
- Neurochemical-vascular theory: suggests abnormal release of vasoactive and pain-inducing substances.
4.2. Other Vestibular and Balance Disorders
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Monkhouse, S. The trigeminal nerve. In Cranial Nerves Functional Anatomy; Monkhouse, S., Ed.; Cambridge University Press: Cambridge, UK, 2006; p. 50e65. [Google Scholar]
- Van der Cruyssen, F.; Politis, C. Neurophysiological aspects of the trigeminal sensory system: An update. Rev. Neurosci. 2018, 29, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D. Neurotransmitters in the vestibular system. Handb. Clin. Neurol. 2016, 137, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, A.; Caradonna, C. The relationship between the stomatognathic system and body posture. Clinics 2009, 64, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Martik, M.L.; Bronner, M.E. Regulatory Logic Underlying Diversification of the Neural Crest. Trends Genet. 2017, 33, 715–727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le Douarin, N.M.; Kalcheim, C. The Neural Crest, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Barlow, L.A. Cranial nerve development: Placodal neurons ride the crest. Curr. Biol. 2002, 12, R171–R173. [Google Scholar] [CrossRef] [PubMed]
- Martik, M.L.; Bronner, M.E. Riding the crest to get a head: Neural crest evolution in vertebrates. Nat. Rev. Neurosci. 2021, 22, 616–626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Méndez-Maldonado, K.; Vega-López, G.A.; Aybar, M.J.; Velasco, I. Neurogenesis From Neural Crest Cells: Molecular Mechanisms in the Formation of Cranial Nerves and Ganglia. Front. Cell Dev. Biol. 2020, 8, 635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurosaka, H.; Trainor, P.A.; Leroux-Berger, M.; Iulianella, A. Cranial nerve development requires co-ordinated Shh and canonical Wnt signaling. PLoS ONE 2015, 10, e0120821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freter, S.; Fleenor, S.J.; Freter, R.; Liu, K.J.; Begbie, J. Cranial neural crest cells form corridors prefiguring sensory neuroblast migration. Development 2013, 140, 3595–3600. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Crockett, D.P.; Nowakowski, R.S.; Gale, N.W.; Zhou, R. Distribution of EphA5 receptor protein in the developing and adult mouse nervous system. J. Comp. Neurol. 2009, 514, 310–328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Northcutt, R.G.; Gans, C. The genesis of neural crest and epidermal placodes: A reinterpretation of vertebrate origins. Q. Rev. Biol. 1983, 58, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, T.T. Development of the inner ear. Curr. Opin. Genet. Dev. 2015, 32, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.K.; Bronner-Fraser, M. Competence, specification and commitment in otic placode induction. Development 2000, 127, 3489–3499. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Groves, A.K. Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development 2006, 133, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Pan, N.; Jahan, I.; Elliott, K.L. Inner ear development: Building a spiral ganglion and an organ of Corti out of unspecified ectoderm. Cell Tissue Res. 2015, 361, 7–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bok, J.; Dolson, D.K.; Hill, P.; Rüther, U.; Epstein, D.J.; Wu, D.K. Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development 2007, 134, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Basch, M.L.; Mishina, Y.; Lyons, K.M.; Segil, N.; Groves, A.K. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. 2010, 30, 15044–15051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wright, T.J.; Mansour, S.L. Fgf3 and Fgf10 are required for mouse otic placode induction. Development 2003, 130, 3379–3390. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Xu, J.; Xu, P.X. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development 2012, 139, 1965–1977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohyama, T. Unraveling inner ear induction by gene manipulation using Pax2-Cre BAC transgenic mice. Brain Res. 2009, 1277, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.S.; Fritzsch, B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS ONE 2013, 8, e62046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pauley, S.; Lai, E.; Fritzsch, B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev. Dyn. 2006, 235, 2470–2482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geng, F.S.; Abbas, L.; Baxendale, S.; Holdsworth, C.J.; Swanson, A.G.; Slanchev, K.; Hammerschmidt, M.; Topczewski, J.; Whitfield, T.T. Semicircular canal morphogenesis in the zebrafish inner ear requires the function of gpr126 (lauscher), an adhesion class G protein-coupled receptor gene. Development 2013, 140, 4362–4374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rakowiecki, S.; Epstein, D.J. Divergent roles for Wnt/β-catenin signaling in epithelial maintenance and breakdown during semicircular canal formation. Development 2013, 140, 1730–1739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vass, Z.; Shore, S.E.; Nuttall, A.L.; Miller, J.M. Direct evidence of trigeminal innervation of the cochlear blood vessels. Neuroscience 1998, 84, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.E.; Vass, Z.; Wys, N.L.; Altschuler, R.A. Trigeminal ganglion innervates the auditory brainstem. J. Comp. Neurol. 2000, 419, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Lefeldt, N.; Heyers, D.; Schneider, N.-L.; Engels, S.; Elbers, D.; Mouritsen, H. Magnetic field-driven induction of ZENK in the trigeminal system of pigeons (Columba livia). J. R. Soc. Interface 2014, 11, 20140777. [Google Scholar] [CrossRef]
- Marfurt, C.F.; Rajchert, D.M. Trigeminal primary afferent projections to “non-trigeminal” areas of the rat central nervous system. J. Comp. Neurol. 1991, 303, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Torvik, A. Merent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures. J. Comp. Neurol. 1956, 106, 51–141. [Google Scholar] [CrossRef]
- Pfaller, K.; Arvidsson, J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J. Comp. Neurol. 1988, 268, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Pinganaud, G.; Bourcier, F.; Buisseret-Delmas, C.; Buisseret, P. Primary trigeminal afferents to the vestibular nuclei in the rat: Existence of a collateral projection to the vestibulo-cerebellum. Neurosci. Lett. 1999, 264, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Valla, J.; Delfini, C.; Diagne, M.; Pinganaud, G.; Buisseret, P.; Buisseret-Delmas, C. Vestibulotrigeminal and vestibulospinal projections in rats: Retrograde tracing coupled to glutamic acid decarboxylase immunoreactivity. Neurosci. Lett. 2003, 340, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Matesz, C.; Kovalecz, G.; Veress, G.; Deák, A.; Rácz, E.; Bácskai, T. Vestibulotrigeminal pathways in the frog, Rana esculenta. Brain Res. Bull. 2008, 75, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Birinyi, A.; Rácz, N.; Kecskes, S.; Matesz, C.; Kovalecz, G. Neural circuits underlying jaw movements for the prey-catching behavior in frog: Distribution of vestibular afferent terminals on motoneurons supplying the jaw. Brain Struct. Funct. 2018, 223, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Giaconi, E.; Deriu, F.; Tolu, E.; Cuccurazzu, B.; Yates, B.J.; Billig, I. Transneuronal tracing of vestibulo-trigeminal pathways innervating the masseter muscle in the rat. Exp. Brain Res. 2006, 171, 330–339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrick, J.L.; Keifer, J. Central trigeminal and posterior eighth nerve projections in the turtle Chrysemys picta studied in vitro. Brain Behav. Evol. 1998, 51, 183–201. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, V.; Tramonti Fantozzi, M.P.; Cataldo, E.; Barresi, M.; Bruschini, L.; Faraguna, U.; Manzoni, D. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Front. Neuroanat. 2018, 11, 130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheridan, N.; Tadi, P. Neuroanatomy, Thalamic Nuclei. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Wijesinghe, R.; Protti, D.A.; Camp, A.J. Vestibular Interactions in the Thalamus. Front. Neural Circuits 2015, 9, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baier, B.; zu Eulenburg, P.; Best, C.; Geber, C.; Müller-Forell, W.; Birklein, F.; Dieterich, M. Posterior insular cortex—Site of vestibular-somato sensory interaction? Brain Behav. 2013, 3, 519–524. [Google Scholar] [CrossRef]
- Kivrak, B.G.; Erzurumlu, R.S. Development of the principal nucleus trigeminal lemniscal projections in the mouse. J. Comp. Neurol. 2013, 521, 299–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, H.; May, P.J.; Dickman, J.D.; Angelaki, D.E. Vestibular signals in primate thalamus: Properties and origins. J. Neurosci. 2007, 27, 13590–13602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Molholm, S.; Sehatpour, P.; Mehta, A.D.; Shpaner, M.; Gomez-Ramirez, M.; Ortigue, S.; Dyke, J.P.; Schwartz, T.H.; Foxe, J.J. Audio-visual multisensory integration in superior parietal lobule revealed by human intracranial recordings. J. Neurophysiol. 2006, 96, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Lang, W.; Büttner-Ennever, J.A.; Büttner, U. Vestibular projections to the monkey thalamus: An autoradiographic study. Brain Res. 1979, 177, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L. Axonal projections and connections of the principal sensory trigeminal nucleus in the monkey. J. Comp. Neurol. 1975, 163, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Neumann, N.; Fullana, M.A.; Radua, J.; Brandt, T.; Dieterich, M.; Lotze, M. Common neural correlates of vestibular stimulation and fear learning: An fMRI meta-analysis. J. Neurol. 2023, 270, 1843–1856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopez, C.; Blanke, O. The thalamocortical vestibular system in animals and humans. Brain Res. Rev. 2011, 67, 119–146. [Google Scholar] [CrossRef]
- Usman, H.O.; Balaban, C.D. Distribution of 5-HT1F Receptors in Monkey Vestibular and Trigeminal Ganglion Cells. Front. Neurol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Theil, D.; Arbusow, V.; Derfuss, T.; Strupp, M.; Pfeiffer, M.; Mascolo, A.; Brandt, T. Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol. 2001, 11, 408–413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dieterich, M.; Obermann, M.; Celebisoy, N. Vestibular migraine: The most frequent entity of episodic vertigo. J. Neurol. 2016, 263 (Suppl. S1), S82–S89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langhagen, T.; Lehrer, N.; Borggraefe, I.; Heinen, F.; Jahn, K. Vestibular migraine in children and adolescents: Clinical findings and laboratory tests. Front. Neurol. 2015, 5, 292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neuhauser, H.; Leopold, M.; von Brevern, M.; Arnold, G.; Lempert, T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001, 56, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D. Migraine. Lancet 2004, 363, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.R.; Wolff, H.G. Mechanism of migraine headache and action of tartrate. Arch. NeurPsych. 1938, 39, 737–763. [Google Scholar] [CrossRef]
- Moskowitz, M.A. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol. Sci. 1992, 13, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Uddman, R.; Edvinsson, L.; Ekman, R.; Kingman, T.; McCulloch, J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: Trigeminal origin and co-existence with substance P. Neurosci. Lett. 1985, 62, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Vass, Z.; Steyger, P.S.; Hordichok, A.J.; Trune, D.R.; Jancsó, G.; Nuttall, A.L. Capsaicin stimulation of the cochlea and electric stimulation of the trigeminal ganglion mediate vascular permeability in cochlear and vertebro-basilar arteries: A potential cause of inner ear dysfunction in headache. Neuroscience 2001, 103, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D. Migraine, vertigo and migrainous vertigo: Links between vestibular and pain mechanisms. J. Vestib. Res. 2011, 21, 315–321. [Google Scholar] [CrossRef]
- Johnson, G.D. Medical management of migraine-related dizziness and vertigo. Laryngoscope 1998, 108 Pt 2, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Balaban, C.D. Serotonin-induced plasma extravasation in the murine inner ear: Possible mechanism of migraine-associated inner ear dysfunction. Cephalalgia 2006, 26, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.K.; Khalmuratova, R.; Jeon, S.Y.; Kim, J.P.; Park, J.J.; Hur, D.G.; Balaban, C.D. Colocalization of 5-HT1F receptor and calcitonin gene-related peptide in rat vestibular nuclei. Neurosci. Lett. 2009, 465, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Vijayakumar, S.; Dow, S.A.; Holt, J.C.; Jordan, P.M.; Luebke, A.E. Loss of α-Calcitonin Gene-Related Peptide (αCGRP) Reduces Otolith Activation Timing Dynamics and Impairs Balance. Front. Mol. Neurosci. 2018, 11, 289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiaocheng, W.; Zhaohui, S.; Junhui, X.; Lei, Z.; Lining, F.; Zuoming, Z. Expression of calcitonin gene-related peptide in efferent vestibular system and vestibular nucleus in rats with motion sickness. PLoS ONE 2012, 7, e47308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.Y.; Lee, C.H.; Yoo, D.M.; Kwon, M.J.; Kim, J.H.; Kim, J.H.; Park, B.; Lee, H.J.; Choi, H.G. Association Between Meniere Disease and Migraine. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 457–464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baloh, R.W. Neurotology of migraine. Headache 1997, 37, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Burstein, R.; Ashina, M.; Tfelt-Hansen, P. Origin of pain in migraine: Evidence for peripheral sensitisation. Lancet Neurol. 2009, 8, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Charbit, A.R.; Andreou, A.P.; Akerman, S.; Holland, P.R. Neurobiology of migraine. Neuroscience 2009, 161, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lopez, I.; Ishiyama, A.; Baloh, R.W. Can migraine damage the inner ear? Arch. Neurol. 2000, 57, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Von Brevern, M.; Lempert, T. Vestibular migraine. Handb. Clin. Neurol. 2016, 137, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ke, Y.J.; Jing, Y.Y.; Diao, T.X.; Yu, L.S. Migraine and Cochlear Symptoms. Curr. Med. Sci. 2021, 41, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Salt, A.N.; Rask-Andersen, H. Responses of the endolym- phatic sac to perilymphatic injections and withdrawals: Evidence for the presence of a one-way valve. Hear Res. 2004, 191, 90–100. [Google Scholar] [CrossRef]
- Kimura, R.S. Animal models of inner ear vascular distur-bances. Am. J. Otolaryngol. 1986, 7, 130–139. [Google Scholar] [CrossRef]
- Lee, K.S.; Kimura, R.S. Ischemia of the endolymphatic sac. Acta Otolaryngol. 1992, 112, 658–666. [Google Scholar] [CrossRef]
- Marano, E.; Marcelli, V.; Di Stasio, E.; Bonuso, S.; Vacca, G.; Manganelli, F.; Marciano, E.; Perretti, A. Trigeminal stimulation elicits a peripheral vestibular imbalance in migraine patients. Headache 2005, 45, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Berra, L.V.; Armocida, D.; Mastino, L.; Rita, A.D.; Norcia, V.D.; Santoro, A.; Piccirilli, M. Trigeminal Neuralgia Secondary to Intracranial Neoplastic Lesions: A Case Series and Comprehensive Review. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Onoda, K.; Ogasawara, Y.; Hirokawa, Y.; Sashida, R.; Fujiwara, R.; Wakamiya, T.; Michiwaki, Y.; Tanaka, T.; Shimoji, K.; Suehiro, E.; et al. Small vestibular schwannoma presented with trigeminal neuralgia: Illustrative case. J. Neurosurg. Case Lessons 2022, 4, CASE22274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- SHaller, L.; Etienne, E.; Kövari, A.D.; Varoquaux, H.; Urbach, M. Becker, Imaging of neurovascular compression syndromes: Trigeminal neuralgia, hemifacial spasm, vestibular paroxysmia, and glossopharyngeal neuralgia. Am. J. Neuroradiol. 2016, 37, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Bes, A.; Kunkel, R.; Lance, J.W.; Nappi, G.; Pfaffenrath, V.; Rose, F.C.; Schoenberg, B.S.; Soyka, D.; Tfelt-Hansen, P.; et al. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- TBrandt, M.; Strupp, M. Dieterich, Vestibular paroxysmia: A treatable neurovascular cross-compression syndrome. J. Neurol. 2016, 263 (Suppl. S1), S90–S96. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4833786/ (accessed on 20 October 2025). [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faralli, M.; Santopietro, G.; Frati, F.; Califano, L. Trigeminal Nerve and Vestibular System: Update on Pathophysiological and Clinical Links. Audiol. Res. 2025, 15, 159. https://doi.org/10.3390/audiolres15060159
Faralli M, Santopietro G, Frati F, Califano L. Trigeminal Nerve and Vestibular System: Update on Pathophysiological and Clinical Links. Audiology Research. 2025; 15(6):159. https://doi.org/10.3390/audiolres15060159
Chicago/Turabian StyleFaralli, Mario, Giuseppe Santopietro, Francesco Frati, and Luigi Califano. 2025. "Trigeminal Nerve and Vestibular System: Update on Pathophysiological and Clinical Links" Audiology Research 15, no. 6: 159. https://doi.org/10.3390/audiolres15060159
APA StyleFaralli, M., Santopietro, G., Frati, F., & Califano, L. (2025). Trigeminal Nerve and Vestibular System: Update on Pathophysiological and Clinical Links. Audiology Research, 15(6), 159. https://doi.org/10.3390/audiolres15060159






