Preset Hearing Aid Program Selection in Low-Income Communities: A Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.3.1. CHW Recruitment and Training
2.3.2. Participant Recruitment
2.4. Material and Apparatus
2.5. Procedure
2.5.1. Screening and Assessment
2.5.2. Hearing Aid Fitting and Follow-Up
2.5.3. Clinically Recommended Program
2.6. Data Analysis
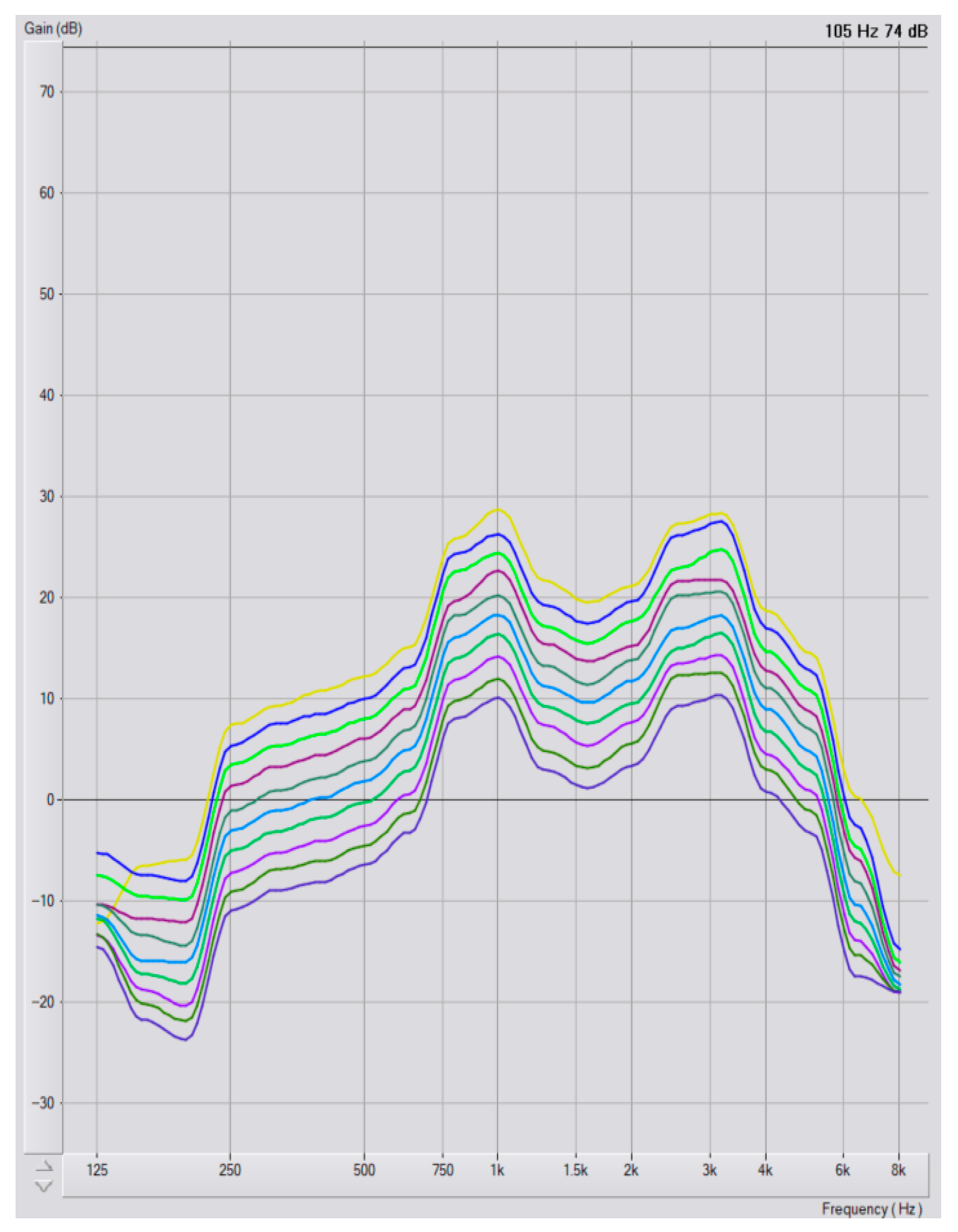
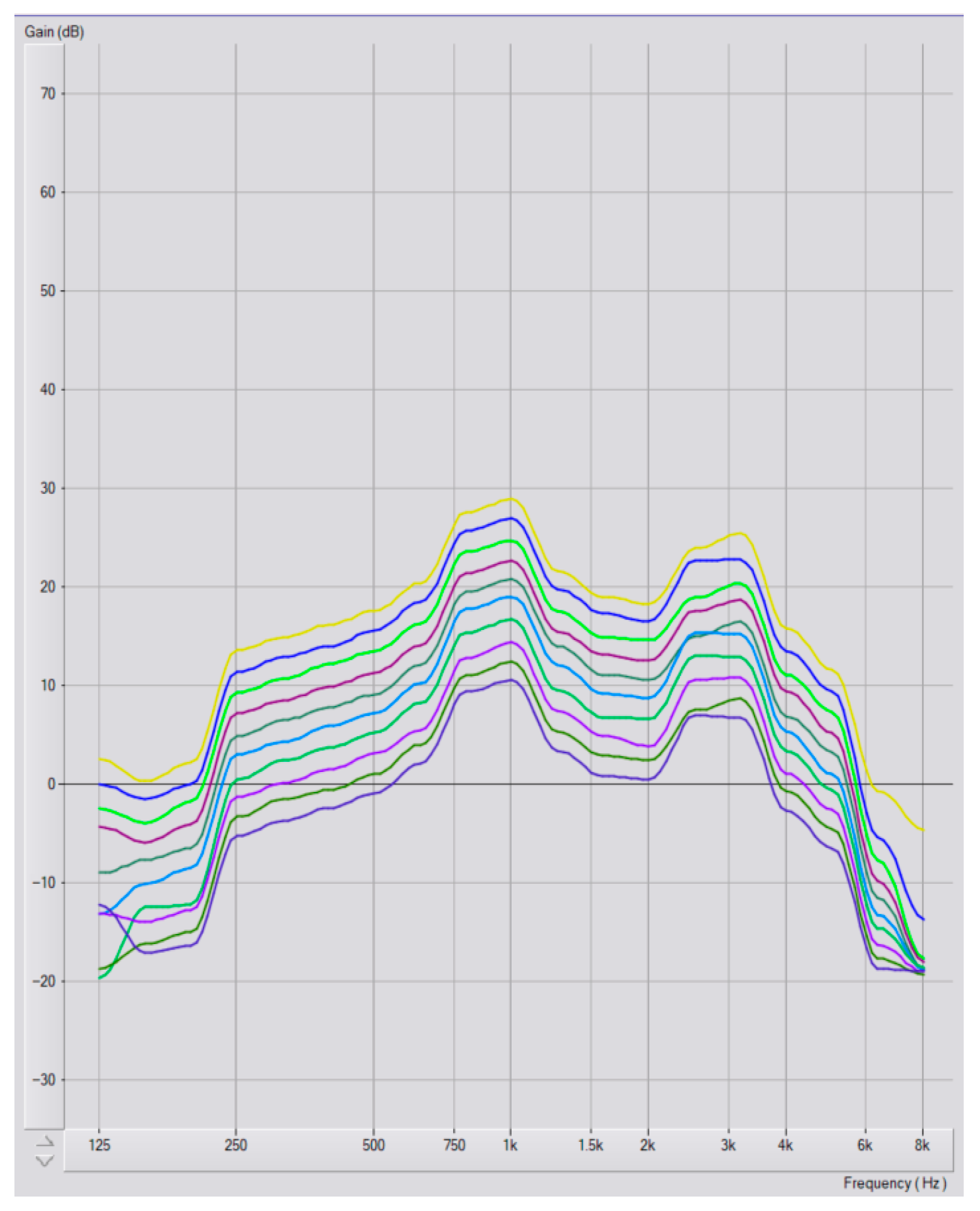
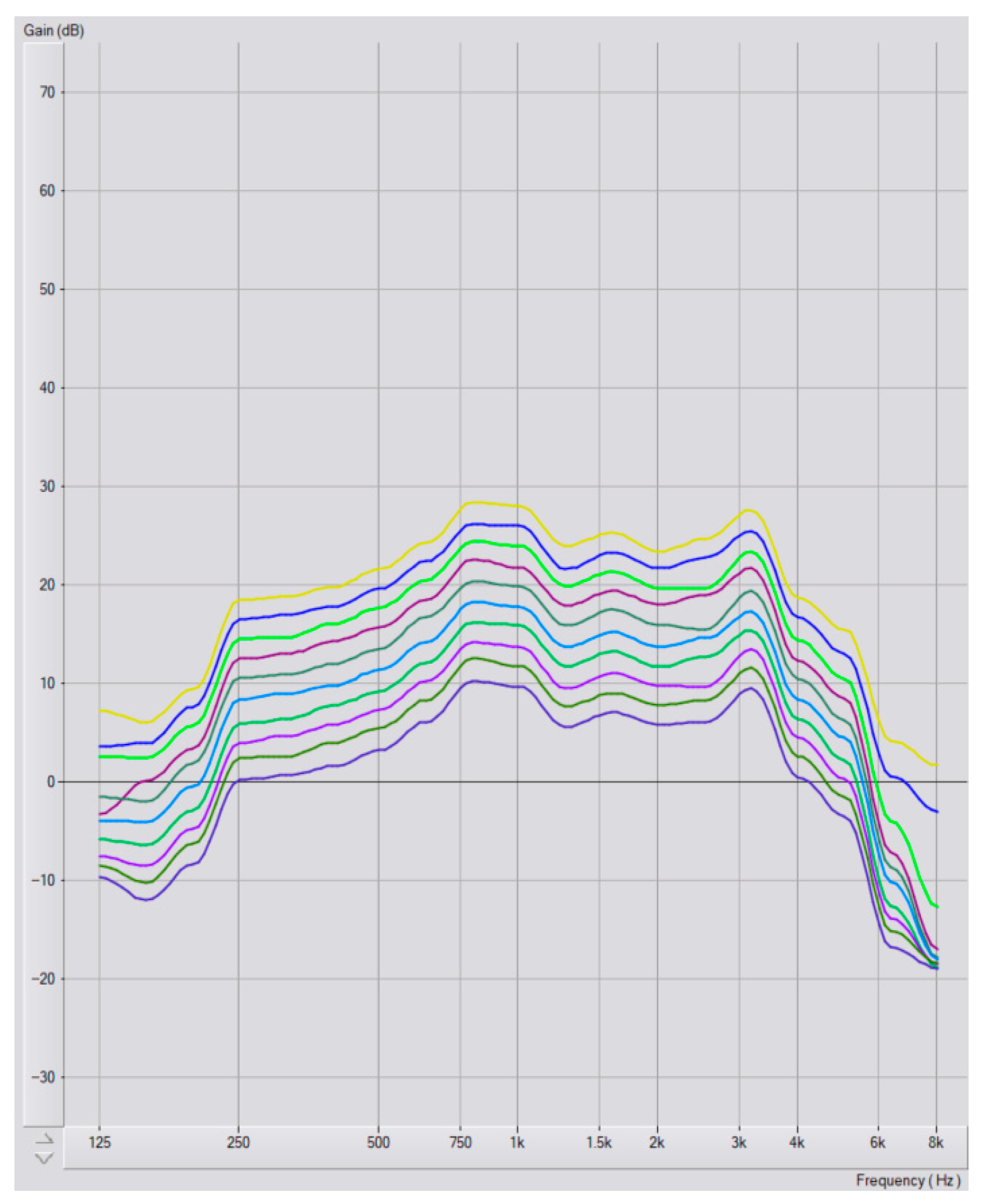
Program Selection and Audiometric Configurations
3. Results
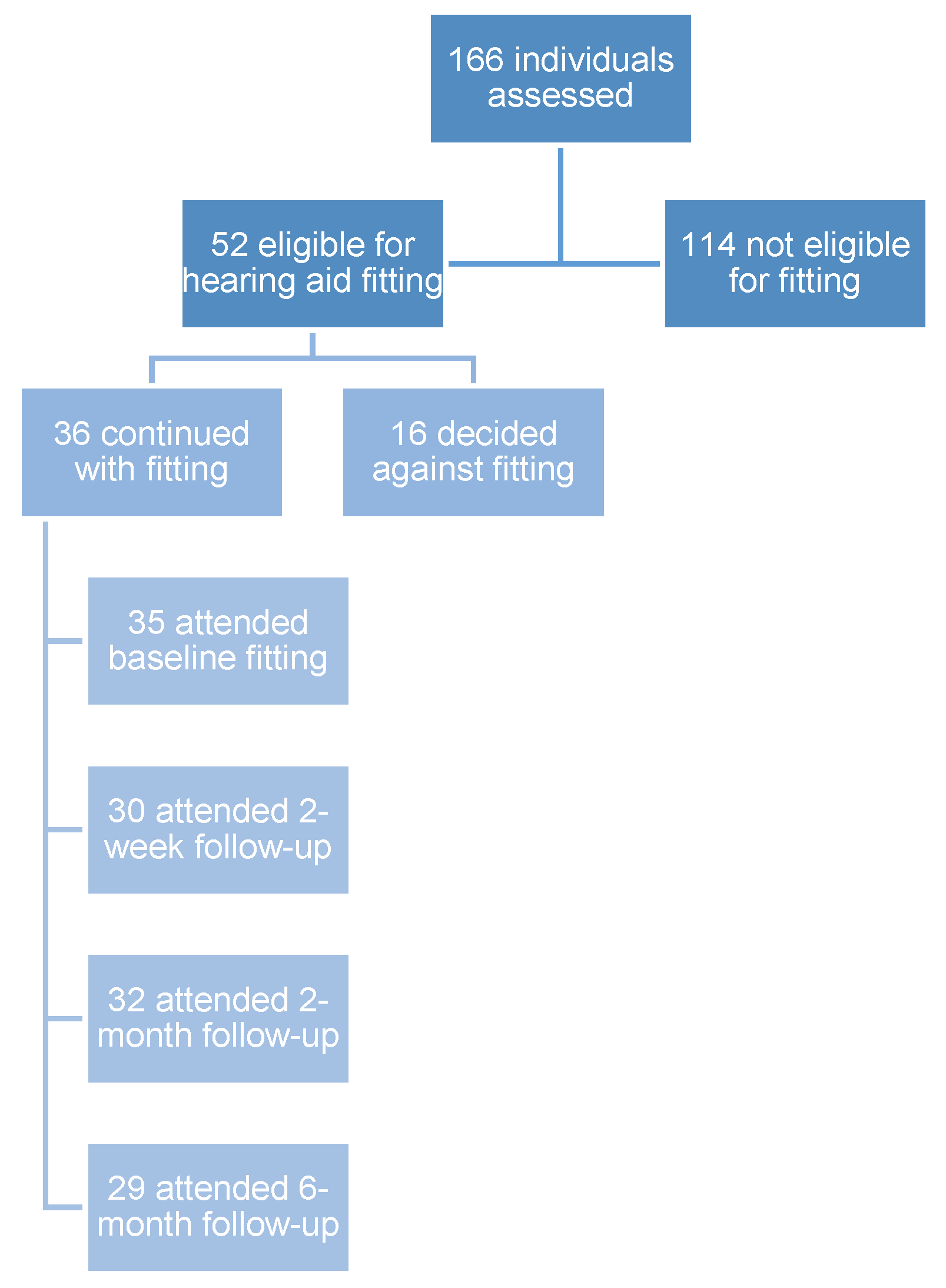
3.1. Recruitment and Demographics
3.2. Hearing Characteristics
3.3. Hearing Aid Program Selection
3.4. Hearing Loss Degree and Program Selection
3.5. Configuration and Program Selection
3.6. Participant Selection vs. Clinically Recommended Program
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4FPTA | Four-Frequency Pure-Tone Average |
| CHW | Community Health Worker |
| EMA-GHAB | Ecological Momentary Assessment Glasgow Hearing Aid Benefit Profile |
| LMI | Low- and Middle-Income |
| mHealth | Mobile Health |
| NGO | Non-Governmental Organization |
| OTC | Over-the-Counter |
| PSAP | Personal Sound Amplification Product |
| PTA | Pure-Tone-Average |
| US | United States |
| WHO | World Health Organization |
Appendix A

References
- World Health Organization. World Report on Hearing; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Bisgaard, N.; Zimmer, S.; Laureyns, M.; Groth, J. A model for estimating hearing aid coverage world-wide using historical data on hearing aid sales. Int. J. Audiol. 2022, 61, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.D. Hearing Loss: Etiology, Management and Societal Implications; Nova Biomedical: Waltham, MA, USA, 2017. [Google Scholar]
- Ganek, H.V.; Madubueze, A.; Merritt, C.E.; Bhutta, Z.A. Prevalence of hearing loss in children living in low- and middle-income countries over the last 10 years: A systematic review. Dev. Med. Child Neurol. 2023, 65, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Olajide, T.G.; Aremu, K.S.; Esan, O.T.; Dosunmu, A.O.; Raji, M.M. Topical Ear Drop Self-medication Practice among the Ear, Nose, and Throat Patients in Ido Ekiti, Nigeria: A Cross-Sectional Study. Ann. Afr. Med. 2018, 17, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Mulwafu, W.; Ensink, R.; Kuper, H.; Fagan, J. Survey of ENT services in sub-Saharan Africa: Little progress between 2009 and 2015. Glob. Health Action 2017, 10, 1289736. [Google Scholar] [CrossRef]
- Chikuse, E.; Jacobs, D.; Banda, A.; Toman, J.; Vallario, J.; Curtis, D.; Porterfield, J.Z. Knowledge, Beliefs, and Treatment Practices for Otitis Media in Malawi: A Community-Based Assessment. Audiol. Res. 2025, 15, 38. [Google Scholar] [CrossRef]
- Kamenov, K.; Martinez, R.; Kunjumen, T.; Chadha, S. Ear and hearing care workforce: Current status and its implications. Ear Hear. 2021, 42, 249–257. [Google Scholar] [CrossRef]
- Swanepoel, D.W. eHealth technologies enable more accessible hearing care. Semin. Hear. 2020, 41, 133–140. [Google Scholar] [CrossRef]
- Swanepoel, D.W. Advancing Equitable Hearing Care: Innovations in Technology and Service Delivery. Folia Phoniatr. Logop. 2023, 75, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.; Jacobs, J.A.; Noble, W.; Bush, M.L.; Snell-Rood, C. Rural Adult Perspectives on Impact of Hearing Loss and Barriers to Care. J. Community Health Publ. Health Promot. Dis. Prev. 2019, 44, 668–674. [Google Scholar] [CrossRef]
- Waterworth, C.J.; Marella, M.; O’Donovan, J.; Bright, T.; Dowell, R.; Bhutta, M.F. Barriers to access to ear and hearing care services in low-and middle-income countries: A scoping review. Glob. Public Health 2022, 17, 3869–3893. [Google Scholar] [CrossRef]
- Mukadam, N.; Sommerlad, A.; Huntley, J.; Livingston, G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: An analysis using cross-sectional survey data. Lancet Glob. Health 2019, 7, e596–e603. [Google Scholar] [CrossRef] [PubMed]
- Brodie, A.; Smith, B.; Ray, J. The impact of rehabilitation on quality of life after hearing loss: A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Gillard, D.M.; Harris, J.P. Cost-effectiveness of Stapedectomy vs Hearing Aids in the Treatment of Otosclerosis. JAMA Otolaryngol.–Head Neck Surg. 2020, 146, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, N.; Ruf, S. Findings from EuroTrak surveys from 2009 to 2015: Hearing loss prevalence, hearing aid adoption, and benefits of hearing aid use. Am. J. Audiol. 2017, 26, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Govender, T.; Barnes, J.M. The Health Status and Unmet Health Needs of Old-Age Pensioners Living in Selected Urban Poor Communities in Cape Town, South Africa. J. Community Health 2014, 39, 1063–1070. [Google Scholar] [CrossRef]
- World Health Organization. Primary Ear and Hearing Care Training Manual; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- World Health Organization. Hearing Aid Service Delivery Approaches for Low- and Middle-Income Settings; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Bright, T.; Mulwafu, W.; Phiri, M.; Ensink, R.J.; Smith, A.; Yip, J.; Mactaggart, I.; Polack, S. Diagnostic accuracy of non-specialist versus specialist health workers in diagnosing hearing loss and ear disease in Malawi. Trop. Med. Int. Health 2019, 24, 817–828. [Google Scholar] [CrossRef]
- Suen, J.J.; Bhatnagar, K.; Emmett, S.D.; Marrone, N.; Robler, S.K.; Swanepoel, D.W.; Wong, A.; Nieman, C.L. Hearing care across the life course provided in the community. Bull. World Health Organ. 2019, 97, 681. [Google Scholar] [CrossRef] [PubMed]
- Yousuf Hussein, S.; Swanepoel, D.W.; Biagio de Jager, L. Smartphone hearing screening in mHealth assisted community-based primary care. J. Telemed. Telecare 2016, 22, 405–412. [Google Scholar] [CrossRef]
- Frisby, C.; Eikelboom, R.H.; Mahomed-Asmail, F.; Kuper, H.; De Kock, T.; Manchaiah, V.; Swanepoel, D.W. Community-based adult hearing care provided by community healthcare workers using mHealth technologies. Glob. Health Action 2022, 15, 2095784. [Google Scholar] [CrossRef]
- Nieman, C.L.; Betz, J.; Garcia Morales, E.E.; Suen, J.J.; Trumbo, J.; Marrone, N.; Han, H.-R.; Szanton, S.L.; Lin, F.R. Effect of a Community Health Worker-Delivered Personal Sound Amplification Device on Self-Perceived Communication Function in Older Adults With Hearing Loss: A Randomized Clinical Trial. JAMA 2022, 328, 2324–2333. [Google Scholar] [CrossRef]
- Borg, J.; Ekman, B.O.; Östergren, P.-O. Is centre-based provision of hearing aids better than community-based provision? A cluster-randomized trial among adolescents in Bangladesh. Disabil. Rehabil. Assist. Technol. 2018, 13, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Mothemela, B.; Caitlin, F.; Mahomed-Asmail, F.; de Kock, T.; Moore, D.R.; Manchaiah, V.; Swanepoel, D.W. Community-based hearing aid fitting model for adults in low-income communities facilitated by community health workers: A feasibility study. Glob. Health Action 2025, 18, 2545630. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preferred Profile for Hearing-Aid Technology Suitable for Low- and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Keidser, G.; Convery, E. Self-fitting hearing aids: Status quo and future predictions. Trends Hear. 2016, 20, 2331216516643284. [Google Scholar] [CrossRef]
- Swanepoel, D.W.; Oosthuizen, I.; Graham, M.A.; Manchaiah, V. Comparing hearing aid outcomes in adults using over-the-counter and hearing care professional service delivery models. Am. J. Audiol. 2023, 32, 314–322. [Google Scholar] [CrossRef]
- Boisvert, I.; Dunn, A.G.; Lundmark, E.; Smith-Merry, J.; Lipworth, W.; Willink, A.; Hughes, S.E.; Nealon, M.; Calvert, M. Disruptions to the hearing health sector. Nat. Med. 2023, 29, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Manchaiah, V.; Swanepoel, D.W.; Sharma, A. Prioritizing research on over-the-counter (OTC) hearing aids for age-related hearing loss. Front. Aging 2023, 4, 1105879. [Google Scholar] [CrossRef]
- Manchaiah, V.; Taddei, S.; Bailey, A.; Swanepoel, D.W.; Rodrigo, H.; Sabin, A. A novel consumer-centric metric for evaluating hearing device audio performance. Front. Audiol. Otol. 2024, 2, 1406362. [Google Scholar] [CrossRef]
- Urbanski, D.; Hernandez, H.; Oleson, J.; Wu, Y.-H. Toward a new evidence-based fitting paradigm for over-the-counter hearing aids. Am. J. Audiol. 2021, 30, 43–66. [Google Scholar] [CrossRef]
- Almufarrij, I.; Dillon, H.; Munro, K.J. Do we need audiogram-based prescriptions? A systematic review. Int. J. Audiol. 2023, 62, 500–511. [Google Scholar] [CrossRef]
- Venkitakrishnan, S.; Urbanski, D.; Wu, Y.-H. Efficacy and Effectiveness of Evidence-Based Non-Self-Fitting Presets Compared to Prescription Hearing Aid Fittings and a Personal Sound Amplification Product. Am. J. Audiol. 2024, 33, 31–54. [Google Scholar] [CrossRef]
- Wu, Y.H.; Stangl, E.; Branscome, K.; Oleson, J.; Ricketts, T. Hearing Aid Service Models, Technology, and Patient Outcomes: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2025, 151, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Portelli, D.; Loteta, S.; Galletti, C.; D’Angelo, M.; Ciodaro, F. Open-fitting hearing aids: A comparative analysis between open behind-the-ear and open completely-in-the-canal instant-fit devices. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 6009–6019. [Google Scholar] [CrossRef]
- Manchaiah, V.; Dhar, S.; Humes, L.; Sharma, A.; Taylor, B.; Swanepoel, D.W. Is There Incremental Benefit with Incremental Hearing Device Technology for Adults with Hearing Loss? Audiol. Res. 2025, 15, 52. [Google Scholar] [CrossRef]
- Wong, L.L. Evidence on self-fitting hearing aids. Trends Amplif. 2011, 15, 215–225. [Google Scholar] [CrossRef]
- Statistics South Africa: City of Cape Town. Available online: https://www.statssa.gov.za/?page_id=4286&id=328 (accessed on 14 July 2025).
- Statistics South Africa: Drakenstein Municipality. Available online: https://www.statssa.gov.za/?page_id=4286&id=90 (accessed on 14 July 2025).
- Lelope, L.A. The Socio-Economic Effects of Xenophobic Attacks on Refugee Entrepreneurs in Atteridgeville; Department of Social Work and Criminology, University of Pretoria: Pretoria, South Africa, 2019. [Google Scholar]
- Van der Spuy, E. The Khayelitsha commission of inquiry: Perceptions and experiences among senior police personnel. Acta Criminol. Afr. J. Criminol. Vict. 2016, 29, 113–128. [Google Scholar]
- Lund, C.; Schneider, M.; Garman, E.C.; Davies, T.; Munodawafa, M.; Honikman, S.; Bhana, A.; Bass, J.; Bolton, P.; Dewey, M.; et al. Task-sharing of psychological treatment for antenatal depression in Khayelitsha, South Africa: Effects on antenatal and postnatal outcomes in an individual randomised controlled trial. Behav. Res. Ther. 2020, 130, 103466. [Google Scholar] [CrossRef]
- Smith, M.E.; Blaauw, D.; Schenck, R. “Sometimes you are Working, Sometimes you are not Working”: Day Labourers’ Battle against Poverty and Vulnerability: The Case of Day Labourers from Mbekweni (Western Cape Province). Afr. J. Dev. Stud. 2021, 11, 195–218. [Google Scholar] [CrossRef]
- Manganye, S.M.; Frisby, C.; Reddy, T.M.; de Kock, T.; Swanepoel, D.W. Hearing loss characteristics and cerumen management efficacy in low-income South African communities: A cross-sectional study. Prim. Health Care Res. Dev. 2025, 26, e27. [Google Scholar] [CrossRef]
- ISO 389-9:2014; Acoustics—Reference Zero for the Calibration of Audiometric Equipment—Part 9: Preferred Test Conditions for the Determination of Reference Hearing Threshold Levels. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 389-1:2017; Acoustics—Reference Zero for the Calibration of Audiometric Equipment—Part 1: Reference Equivalent Threshold Sound Pressure Levels for Pure Tones and Supra-Aural Earphones. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 8253-1:2010; Acoustics—Audiometric Test Methods—Part 1: Pure-Tone Air and Bone Conduction Audiometry. International Organization for Standardization: Geneva, Switzerland, 2010.
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pichora-Fuller, M.K.; Hayes, D.; von Schroeder, H.P.; Carnahan, H. The aging hand and the ergonomics of hearing aid controls. Ear Hear. 2013, 34, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Dillon, H. Hearing Aids; Thieme Medical Publishers: New York, NY, USA, 2012. [Google Scholar]
- Wentzel, C.; Swanepoel, D.W.; Mahomed-Asmail, F.; Beukes, E.; Dawes, P.; Munro, K.; Almufarrij, I.; Manchaiah, V. Auditory Acclimatization in New Adult Hearing Aid Users: A Registered Systematic Review of Magnitude, Key Variables, and Clinical Relevance. J. Speech Lang. Hear. Res. (JSLHR) 2025, 68, 3445–3479. [Google Scholar] [CrossRef]
- Perry, T.T.; Nelson, P.B.; Van Tasell, D.J. Listener Factors Explain Little Variability in Self-Adjusted Hearing Aid Gain. Trends Hear. 2019, 23, 2331216519837124. [Google Scholar] [CrossRef]
- Keidser, G.; O’Brien, A.; Carter, L.; McLelland, M.; Yeend, I. Variation in preferred gain with experience for hearing-aid users. Int. J. Audiol. 2008, 47, 621–635. [Google Scholar] [CrossRef]
- Almufarrij, I.; Dillon, H.; Adams, B.; Greval, A.; Munro, K.J. Listening Preferences of New Adult Hearing Aid Users: A Registered, Double-Blind, Randomized, Mixed-Methods Clinical Trial of Initial Versus Real-Ear Fit. Trends Hear. 2023, 27, 23312165231189596. [Google Scholar] [CrossRef] [PubMed]
- Knoetze, M.; Manchaiah, V.; Cormier, K.; Schimmel, C.; Sharma, A.; Swanepoel, D.W. Gain Analysis of Self-Fitting Over-the-Counter Hearing Aids: A Comparative and Longitudinal Analysis. Audiol. Res. 2025, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yun, D.; Liu, Y. Individualized estimation of the Speech Intelligibility Index for short sentences: Test-retest reliability. J. Acoust. Soc. Am. 2020, 148, 1647. [Google Scholar] [CrossRef] [PubMed]
- Yoho, S.E.; Bosen, A.K. Individualized frequency importance functions for listeners with sensorineural hearing loss. J. Acoust. Soc. Am. 2019, 145, 822. [Google Scholar] [CrossRef]
- Gößwein, J.A.; Rennies, J.; Huber, R.; Bruns, T.; Hildebrandt, A.; Kollmeier, B. Evaluation of a semi-supervised self-adjustment fine-tuning procedure for hearing aids. Int. J. Audiol. 2023, 62, 159–171. [Google Scholar] [CrossRef]
- Søgaard Jensen, N.; Hau, O.; Bagger Nielsen, J.B.; Bundgaard Nielsen, T.; Vase Legarth, S. Perceptual Effects of Adjusting Hearing-Aid Gain by Means of a Machine-Learning Approach Based on Individual User Preference. Trends Hear. 2019, 23, 2331216519847413. [Google Scholar] [CrossRef]
- Frisby, C.; Eikelboom, R.H.; Mahomed-Asmail, F.; Kuper, H.; Moore, D.R.; de Kock, T.; Manchaiah, V.; Swanepoel, D.W. Mobile Health Hearing Aid Acclimatization and Support Program in Low-Income Communities: Feasibility Study. JMIR Form. Res. 2023, 7, e46043. [Google Scholar] [CrossRef]
- Humes, L.E.; Rogers, S.E.; Quigley, T.M.; Main, A.K.; Kinney, D.L.; Herring, C. The effects of service-delivery model and purchase price on hearing-aid outcomes in older adults: A randomized double-blind placebo-controlled clinical trial. Am. J. Audiol. 2017, 26, 53–79. [Google Scholar] [CrossRef] [PubMed]
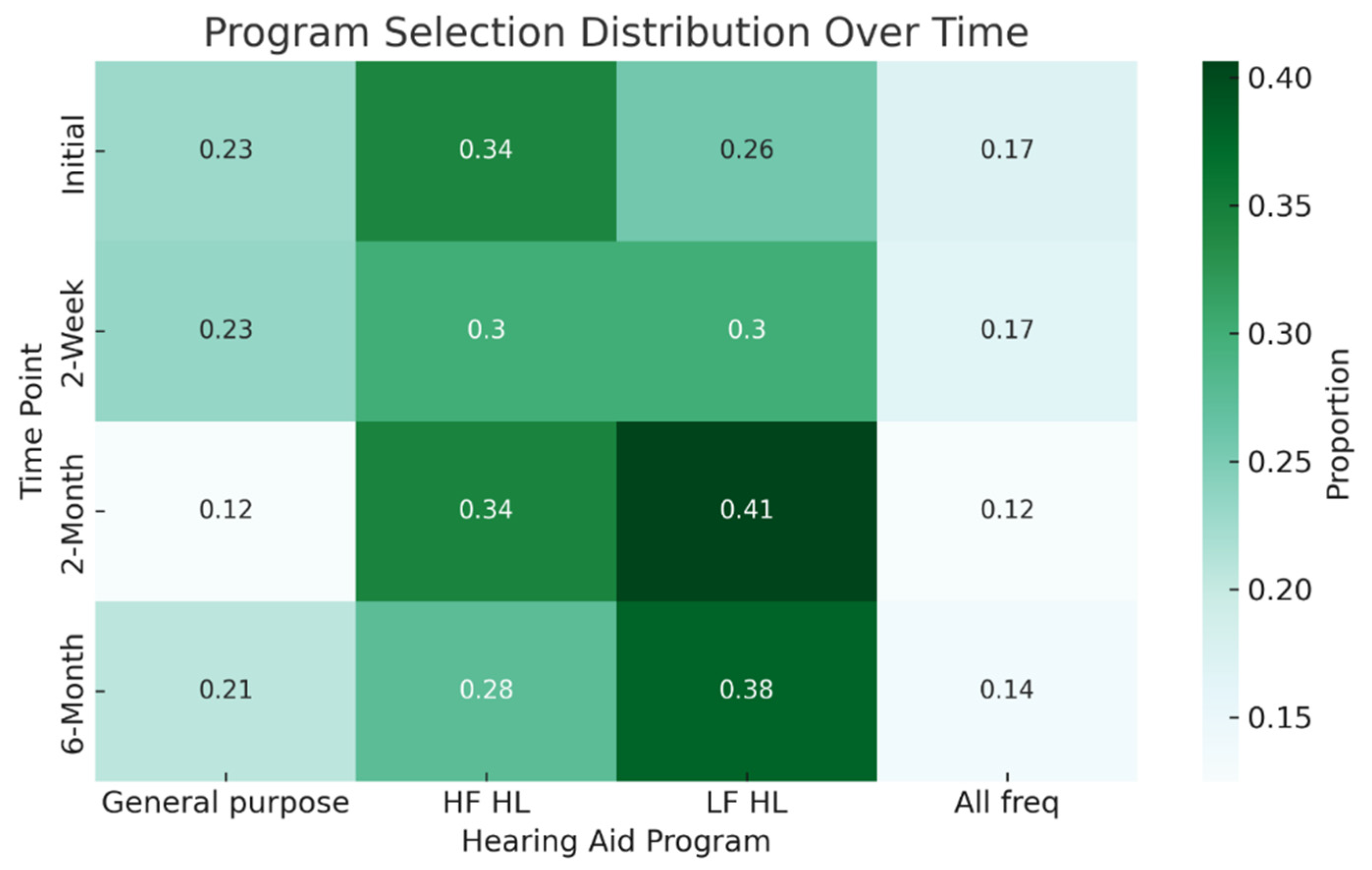


| General Purpose % (n) | High Frequency HL % (n) | Low Frequency HL % (n) | All Frequencies % (n) | |
|---|---|---|---|---|
| Clinical recommendation | - | 58.3 (21) | - | 41.7 (15) |
| Participant selection | ||||
| Initial | 22.9 (8) | 34.3 (12) | 25.7 (9) | 17.1 (6) |
| 2-week | 23.3 (7) | 30.0 (9) | 30.0(9) | 16.7 (5) |
| 2-month | 12.5 (4) | 34.1 (11) | 40.6 (13) | 12.5 (4) |
| 6-month | 20.7 (6) | 27.6 (8) | 37.9 (11) | 13.8 (4) |
| Right Ears % (n) | Left Ears % (n) | |
|---|---|---|
| Degree (4FPTA) | ||
| Moderate (35–49 dB HL) | 55.6 (20) | 55.6 (20) |
| Moderately severe (50–64 dB HL) | 36.1 (13) | 33.3 (12) |
| Severe (65–79 dB HL) | 8.3 (3) | 11.1 (4) |
| Configuration * | ||
| High-frequency loss | 52.8 (19) | 58.3 (21) |
| Flat loss | 47.2 (17) | 38.9 (14) |
| Low-frequency loss | 0 (0) | 2.8 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croucamp, A.; Frisby, C.; Manchaiah, V.; de Kock, T.; Swanepoel, D.W. Preset Hearing Aid Program Selection in Low-Income Communities: A Longitudinal Study. Audiol. Res. 2025, 15, 137. https://doi.org/10.3390/audiolres15050137
Croucamp A, Frisby C, Manchaiah V, de Kock T, Swanepoel DW. Preset Hearing Aid Program Selection in Low-Income Communities: A Longitudinal Study. Audiology Research. 2025; 15(5):137. https://doi.org/10.3390/audiolres15050137
Chicago/Turabian StyleCroucamp, Anné, Caitlin Frisby, Vinaya Manchaiah, Tersia de Kock, and De Wet Swanepoel. 2025. "Preset Hearing Aid Program Selection in Low-Income Communities: A Longitudinal Study" Audiology Research 15, no. 5: 137. https://doi.org/10.3390/audiolres15050137
APA StyleCroucamp, A., Frisby, C., Manchaiah, V., de Kock, T., & Swanepoel, D. W. (2025). Preset Hearing Aid Program Selection in Low-Income Communities: A Longitudinal Study. Audiology Research, 15(5), 137. https://doi.org/10.3390/audiolres15050137







