The Predictive Role of Video Head Impulse Testing Patterns of Anti-Compensatory Saccades Using the Suppression Head Impulse Paradigm for the Diagnosis of Mild Acute Unilateral Vestibular Loss
Abstract
1. Introduction
2. Materials and Methods
- Dizziness (not vertigo).
- Nausea (not vomiting).
- Unsteadiness and non-rotational vertigo (not a fall).
- Slight unidirectional rotation on the Unterberger test (less than 30 degrees of rotation in 50 steps).
- Positive past-pointing test (unilateral deviation on the Barany outstretched arms test).
- No evidence of spontaneous nystagmus (infrared video goggles evaluation).
- Slight rhythmic, conjugated, persistent (not paroxysmal), horizontal, unidirectional nystagmus on positioning testing (infrared video goggles evaluation).
- Nystagmus inhibited by fixation (infrared video goggles evaluation).
- Positive HIT (head impulse test) outcomes were considered doubtful.
- Negative HST (infrared video goggles evaluation).
- Borderline lower VOR gain values for the affected side (as is known, normal VOR gain values for the horizontal canal are 0.8–1.0; we considered “lower borderline VOR gain values” to be 10% less than 0.8, which means a range of 0.72–0.8).
- Borderline asymmetry of the VOR gain values on the vHIT in HIMP mode was observed (we considered all cases with less than a 20% discrepancy between the two sides, even when the VOR gain was within the normal range of 0.8–1.0).
- Long-lasting vomiting.
- Rotational vertigo.
- Falls, ataxia, and dysmetria.
- Unterberger test: Rotation of more than 30 degrees in 50 steps.
- Spontaneous first-, second-, and third-degree nystagmus in the sitting position.
- Clearly positive HIT.
- HST positivity (infrared video goggles evaluation).
- Simultaneous diagnosis of BPPV (infrared video goggles evaluation).
- Paroxysmal, arrhythmic, deconjugated, or non-inhibited by fixation nystagmus (infrared video goggles evaluation).
- Other signs and/or symptoms of pathologies of central, vascular, metabolic, traumatic, iatrogenic, or malformative origin.
- VHIT TESTING:
- VOR asymmetry using the HIMP of more than 20% on the horizontal semicircular canal (HSC).
- Affected side with HSC VOR gain ≥ 0.8.
- (1)
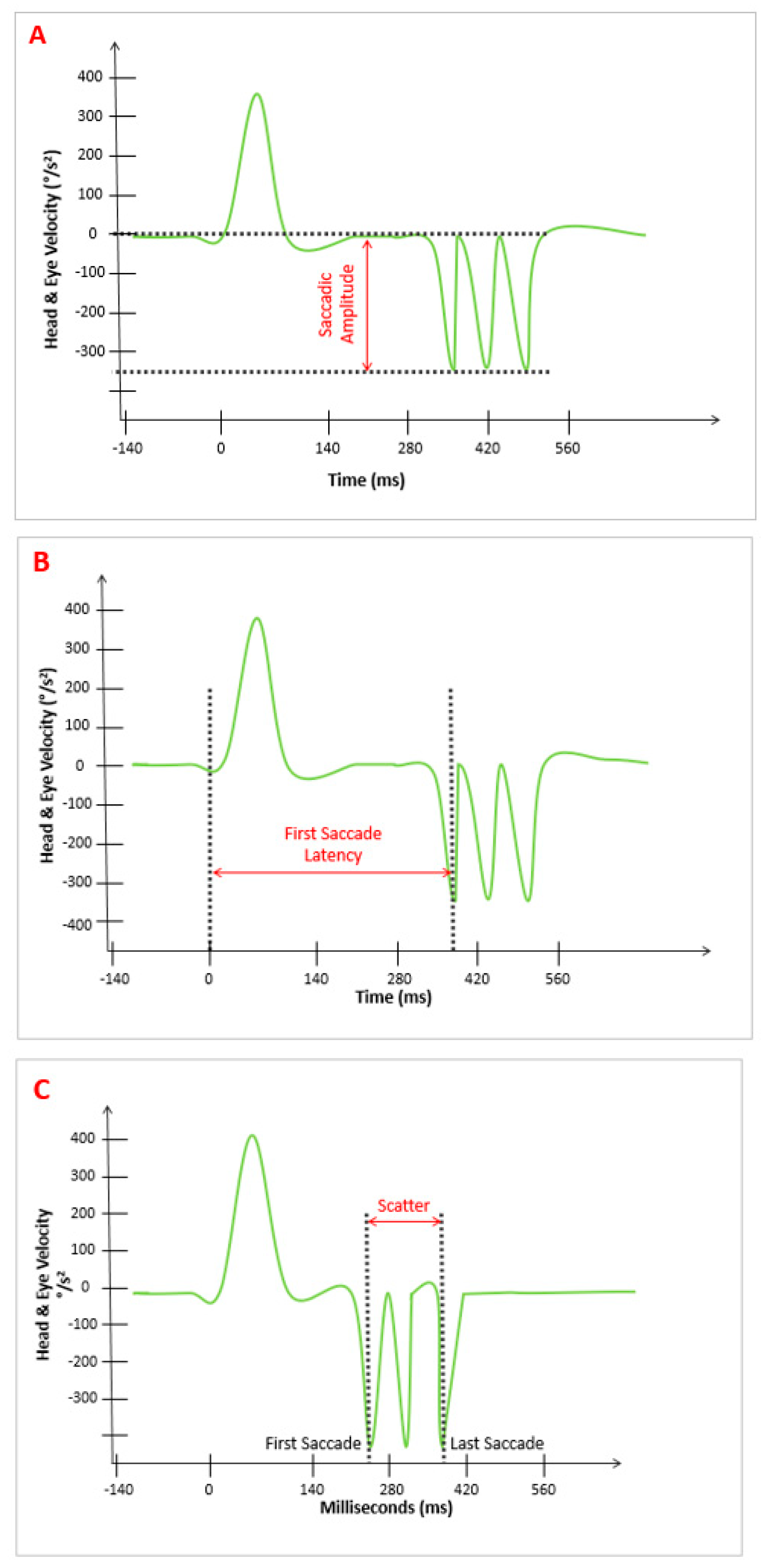
- “Amplitude pattern” (A): The amplitude of the AS that appeared after the SHIMP VOR complex. The device provided the average value of parameter “A,” which was expressed in °/s (Figure 1A).
- (2)
- “Latency pattern” (L): The time at which the first AS appeared after the SHIMP VOR complex started. The average of the values of parameter “L” was provided by the device and was expressed in ms (Figure 1B).
- (3)
- “Scattered pattern” (S): The time duration between the appearance of the first and last AS after the VOR complex on the stimulated side. The average value of parameter “S” was provided by the device and was expressed in ms (Figure 1C).
- (1)
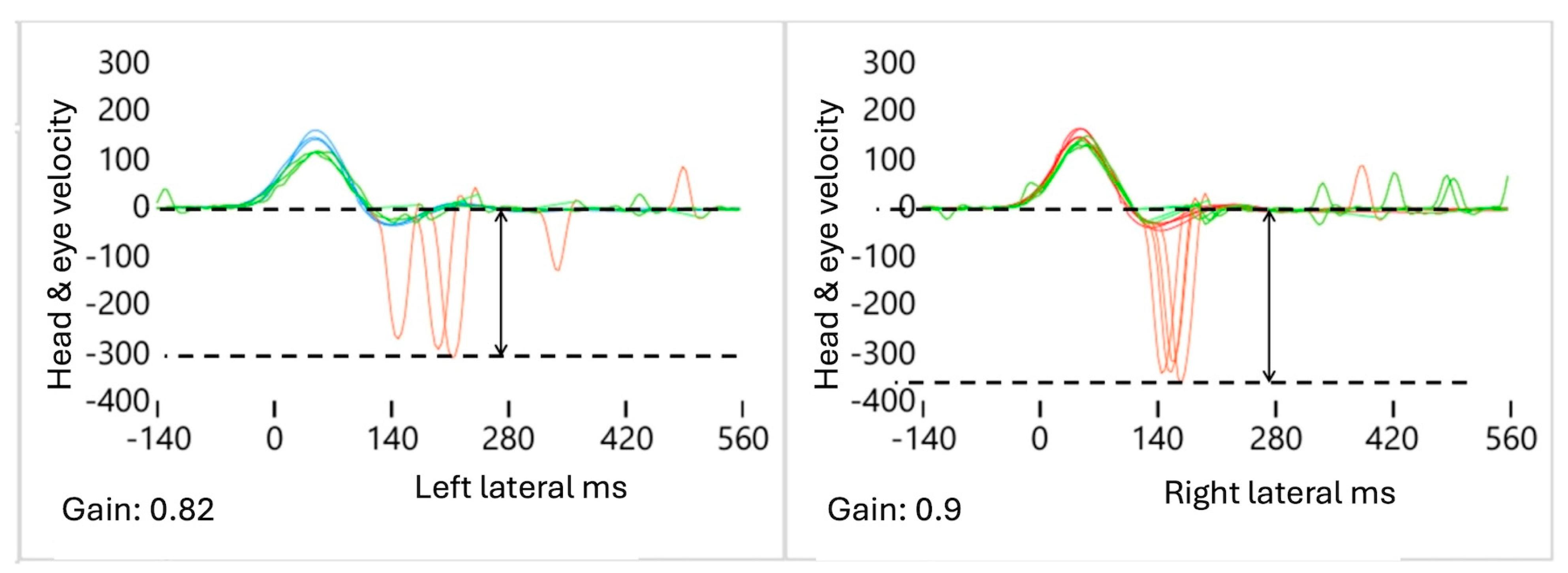
- Decreased A: The ΔA value is defined as the difference in the average AS amplitude between the affected (AFS) and healthy (HTS) sides, calculated using the formula AAFS − AHTS = ΔA. As the VOR gain value decreased, we observed that the amplitude of the “A” values also decreased (Figure 2). In the case of mAUVL, slightly decreased VOR values produced slightly low “A” values that were present but not very sensitive.
- (2)
- Increased S: The degree of the “S” pattern of the AS on both sides is obtained by calculating the difference in the AS latency between the first saccade (FS) and the last saccade (LS) of the AFS, compared to the contralateral HTS. This is calculated using the formula (FSAFS-LSAFS)-(FSHTS-LSHTS), which equals ΔSAFS-ΔSHTS, or “S”. As the VOR gain value decreased in the AFS, even slightly as in mAUVL, we observed that the “S” parameter values increased significantly ipsilaterally compared to the contralateral HTS (Figure 3).
- (3)
- Increased L: The latency time between the start of the SHIMP VOR complex and the FS was significantly increased in the AFS compared to the contralateral HTS. It is calculated using the formula FSAFS − FSHTS = ΔFS (Figure 4).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| °/s | Degree Per Second |
| A | Amplitude Pattern |
| AFS | Affected Side |
| AS | Anti-Compensatory Saccades |
| AUVL | Acute Unilateral Vestibular Loss |
| BPPV | Benign Paroxysmal Positional Vertigo |
| CS | Compensatory Saccade |
| FS | First Saccade |
| HIMP | Head Impulse Paradigm |
| HIT | Head Impulse Test |
| HSs | Healthy Subjects |
| HSC | Horizontal Semicircular Canal |
| HTS | Healthy Side |
| IQR | Interquartile Range |
| L | Latency Pattern |
| LS | Last Saccade |
| mAUVL | Mild Acute Unilateral Vestibular Loss |
| ms | Milliseconds |
| S | Scattered Pattern |
| sAUVL | Severe Acute Unilateral Vestibular Loss |
| SHIMP | Suppression Head Impulse Paradigm |
| vHIT | Video Head Impulse Test |
| VOR | Vestibulo-Ocular Reflex |
References
- Hanley, K.T.O.D.; O’Dowd, T. Symptoms of vertigo in general practice: A prospective study of diagnosis. Br. J. Gen. Pract. 2002, 52, 809–812. [Google Scholar]
- Strupp, M.; Bisdorff, A.; Furman, J.; Hornibrook, J.; Jahn, K.; Maire, R.; Newman-Toker, D.; Magnusson, M. Acute unilateral vestibulopathy/vestibular neuritis: Diagnostic criteria. J. Vestib. Res. 2022, 32, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Sekitani, T.; Imate, Y.; Noguchi, T.; Inokuma, T. Vestibular neuronitis: Epidemiological survey by questionnaire in Japan. Acta Otolaryngol. Suppl. 1993, 503, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, M.; Salerni, L.; Ferretti, F.; Bindi, I.; Gualtieri, G.; Corallo, G.; Viberti, F.; Gusinu, R.; Fantino, C.; Ponzo, S.; et al. The incidence of vestibular neuritis in Italy. Front. Neurol. 2023, 14, 1177621. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, H.K. The epidemiology of dizziness and vertigo. Handb. Clin. Neurol. 2016, 137, 67–82. [Google Scholar] [PubMed]
- Halmagyi, G.M.; Chen, L.; MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Curthoys, I.S. The Video Head. Impulse Test. Front. Neurol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.; Comacchio, F.; Casani, A.P. Trattato Italiano di Vestibologia Clinica; CLEUP Società Cooperativa: Padova, Italy, 2021. [Google Scholar]
- MacDougall, H.G.; McGarvie, L.A.; Halmagyi, G.M.; Rogers, S.J.; Manzari, L.; Burgess, A.M.; Curthoys, I.S.; Weber, K.P. A new saccadic indicator of peripheral vestibular function based on the video head impulse test. Neurology 2016, 87, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; De Angelis, S.; Princi, A.A.; Galeoto, G.; Tramontano, M. The Clinical Use of the Suppression Head Impulse Paradigm in Patients with Vestibulopathy: A Systematic Review. Healthcare 2022, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, J.Y.; Nam, W.; Noh, S.; Chang, S.O.; Kim, M.B. Comparing the Suppression Head Impulse Paradigm and the Head Impulse Paradigm in Vestibular Neuritis. Otol. Neurotol. 2020, 41, e76–e82. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, M.-B. Importance of Video Head Impulse Test Parameters for Recovery of Symptoms in Acute Vestibular Neuritis. Otol. Neurotol. 2020, 41, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Magnani, C.; Sterkers, O.; Lamas, G.; Vidal, P.-P.; Sadoun, J.; Curthoys, I.S.; de Waele, C. Saccadic Velocity in the New Suppression Head Impulse Test: A New Indicator of Horizontal Vestibular Canal Paresis and of Vestibular Compensation. Front. Neurol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Halmagyi, G.M. Central Lesions With Selective Semicircular Canal Involvement Mimicking Bilateral Vestibulopathy. Front. Neurol. 2018, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Halmagyi, G.M. Video Head Impulse Testing: From Bench to Bedside. Semin. Neurol. 2020, 40, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, V.; Weber, K.; Bockisch, C.; Hegemann, S. Compensatory saccades in head impulse testing influence the dynamic visual acuity of patients with unilateral peripheral vestibulopathy1. J. Vestib. Res. 2016, 26, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Mantokoudis, G.; Schubert, M.C.; Tehrani, A.S.S.; Wong, A.L.; Agrawal, Y. Early adaptation and compensation of clinical vestibular responses after unilateral vestibular deafferentation surgery. Otol. Neurotol. 2014, 35, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Allum, J.H.J.; Keshner, E.A.; Honegger, F.; Pfaltz, C.R. Indicators of the influence a peripheral vestibular deficit has on vestibulo-spinal reflex responses controlling postural stability. Acta Otolaryngol. 1988, 106, 252–263. [Google Scholar] [CrossRef] [PubMed]


| Variable | Group 1 | Group 2 | Group 3 | |
|---|---|---|---|---|
| Amplitude—lower VOR side | Mean ± Standard Deviation | −283.3 ± 57.5 | −242.4 ± 63.4 | −309.1 ± 58.2 |
| Median [IQR] | −288.5 [84.0] | −240.5 [72.0] | −307.0 [80.0] | |
| Amplitude—higher VOR side | Mean ± Standard Deviation | −322.8 ± 45.3 | −338.9 ± 54.7 | −331.3 ± 56.5 |
| Median [IQR] | −329.5 [52.8] | −339.5 [41.5] | −329.0 [59.8] | |
| Scatter—lower VOR side | Mean ± Standard Deviation | 176.8 ± 69.0 | 160.5 ± 86.6 | 67.6 ± 34.9 |
| Median [IQR] | 176.0 [100.0] | 164.0 [128.0] | 56.0 [18.0] | |
| Scatter—higher VOR side | Mean ± Standard Deviation | 79.3 ± 62.4 | 87.2 ± 68.5 | 59.8 ± 36.6 |
| Median [IQR] | 64.0 [60.0] | 58.0 [136.0] | 51.0 [15.0] | |
| Latency—lower VOR side | Mean ± Standard Deviation | 184.7 ± 46.7 | 178.0 ± 29.1 | 148.4 ± 12.0 |
| Median [IQR] | 176.0 [46.0] | 176.0 [46.0] | 148.0 [17.2] | |
| Latency—higher VOR side | Mean ± Standard Deviation | 142.4 ± 27.3 | 140.2 ± 21.0 | 136.0 ± 13.1 |
| Median [IQR] | 136.0 [41.0] | 136.0 [34.0] | 132.0 [14.0] |
| Group | Tested Variables | N | Shapiro p (Lower) | Shapiro p (Higher) | Test | Statistic | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | Amplitude—lower vs. higher VOR side | 48 | 0.305 | 0.228 | t-test | 3.74 | 0.0003 |
| 2 | Amplitude—lower vs. higher VOR side | 16 | 0.663 | 0.421 | t-test | 4.61 | <0.0001 |
| 3 | Amplitude—lower vs. higher VOR side | 10 | 0.820 | 0.560 | t-test | 0.87 | 0.398 |
| 1 | Scatter—lower vs. higher VOR side | 61 | 0.323 | 0.000012 | Mann–Whitney U | 3175.0 | <0.0001 |
| 2 | Scatter—lower vs. higher VOR side | 13 | 0.640 | 0.018 | Mann–Whitney U | 131.0 | 0.018 |
| 3 | Scatter—lower vs. higher VOR side | 10 | 0.005 | 0.016 | Mann–Whitney U | 61.5 | 0.404 |
| 1 | Latency—lower vs. higher VOR side | 52 | 0.00029 | 0.00037 | Mann–Whitney U | 2189.5 | <0.0001 |
| 2 | Latency—lower vs. higher VOR side | 19 | 0.235 | 0.165 | t-test | 4.59 | <0.0001 |
| 3 | Latency—lower vs. higher VOR side | 16 | 0.142 | 0.152 | t-test | 2.78 | 0.009 |
| Variable | Group | Test | Statistic | p-Value |
|---|---|---|---|---|
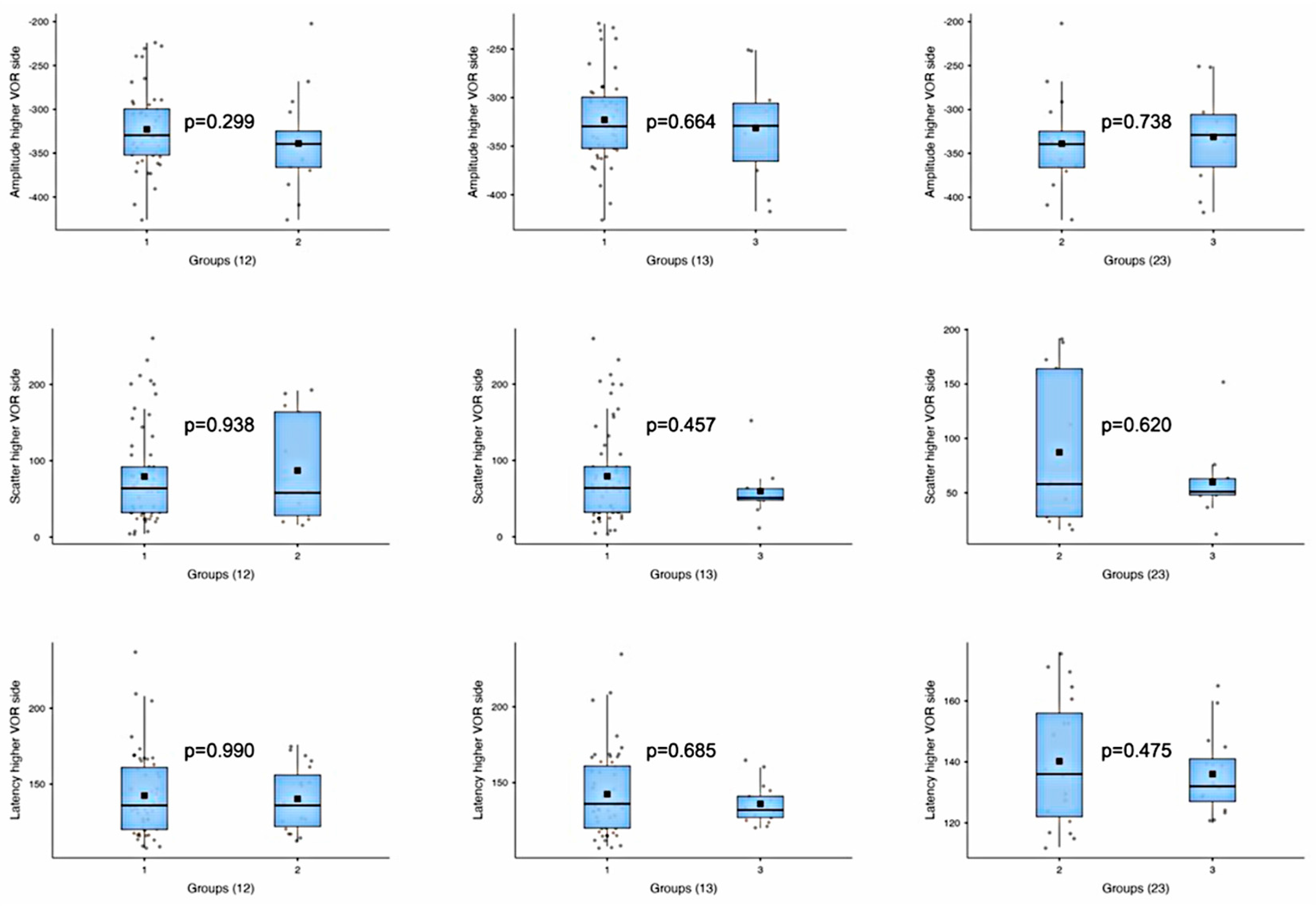
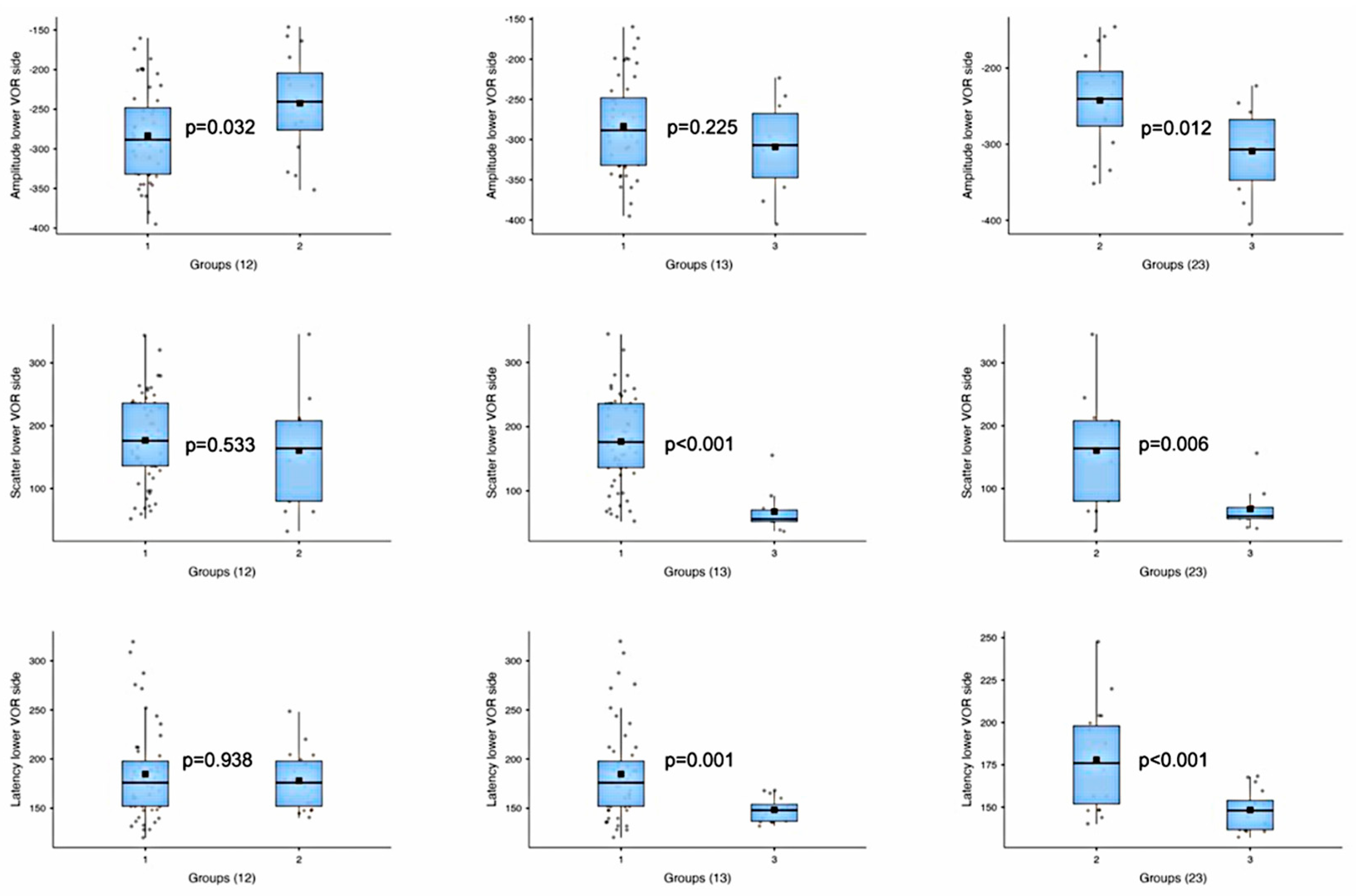
| Amplitude—lower VOR side | 1 vs. 2 | t-test | −2.28 | 0.032 |
| 1 vs. 3 | t-test | 1.28 | 0.225 | |
| 2 vs. 3 | t-test | 2.74 | 0.012 | |
| Amplitude—higher VOR side | 1 vs. 2 | t-test | 1.06 | 0.299 |
| 1 vs. 3 | t-test | 0.45 | 0.664 | |
| 2 vs. 3 | t-test | −0.34 | 0.738 | |
| Scatter—lower VOR side | 1 vs. 2 | t-test | 0.64 | 0.533 |
| 1 vs. 3 | Mann–Whitney U | 566.5 | <0.001 | |
| 2 vs. 3 | Mann–Whitney U | 109.5 | 0.006 | |
| Scatter—higher VOR side | 1 vs. 2 | Mann–Whitney U | 402.5 | 0.938 |
| 1 vs. 3 | Mann–Whitney U | 350.5 | 0.457 | |
| 2 vs. 3 | Mann–Whitney U | 73.5 | 0.620 | |
| Latency—lower VOR side | 1 vs. 2 | Mann–Whitney U | 500.5 | 0.938 |
| 1 vs. 3 | Mann–Whitney U | 645.0 | 0.001 | |
| 2 vs. 3 | t-test | 4.05 | <0.001 | |
| Latency—higher VOR side | 1 vs. 2 | Mann–Whitney U | 492.5 | 0.990 |
| 1 vs. 3 | Mann–Whitney U | 444.5 | 0.685 | |
| 2 vs. 3 | t-test | 0.72 | 0.475 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzanelli, C.; Pontara, F.; Redaelli de Zinis, L.O. The Predictive Role of Video Head Impulse Testing Patterns of Anti-Compensatory Saccades Using the Suppression Head Impulse Paradigm for the Diagnosis of Mild Acute Unilateral Vestibular Loss. Audiol. Res. 2025, 15, 110. https://doi.org/10.3390/audiolres15050110
Balzanelli C, Pontara F, Redaelli de Zinis LO. The Predictive Role of Video Head Impulse Testing Patterns of Anti-Compensatory Saccades Using the Suppression Head Impulse Paradigm for the Diagnosis of Mild Acute Unilateral Vestibular Loss. Audiology Research. 2025; 15(5):110. https://doi.org/10.3390/audiolres15050110
Chicago/Turabian StyleBalzanelli, Cristiano, Fabio Pontara, and Luca Oscar Redaelli de Zinis. 2025. "The Predictive Role of Video Head Impulse Testing Patterns of Anti-Compensatory Saccades Using the Suppression Head Impulse Paradigm for the Diagnosis of Mild Acute Unilateral Vestibular Loss" Audiology Research 15, no. 5: 110. https://doi.org/10.3390/audiolres15050110
APA StyleBalzanelli, C., Pontara, F., & Redaelli de Zinis, L. O. (2025). The Predictive Role of Video Head Impulse Testing Patterns of Anti-Compensatory Saccades Using the Suppression Head Impulse Paradigm for the Diagnosis of Mild Acute Unilateral Vestibular Loss. Audiology Research, 15(5), 110. https://doi.org/10.3390/audiolres15050110






