Role of Lung Function, Chronic Obstructive Pulmonary Disease on Hearing Impairment: Evidence for Causal Effects and Clinical Implications
Abstract
1. Introduction
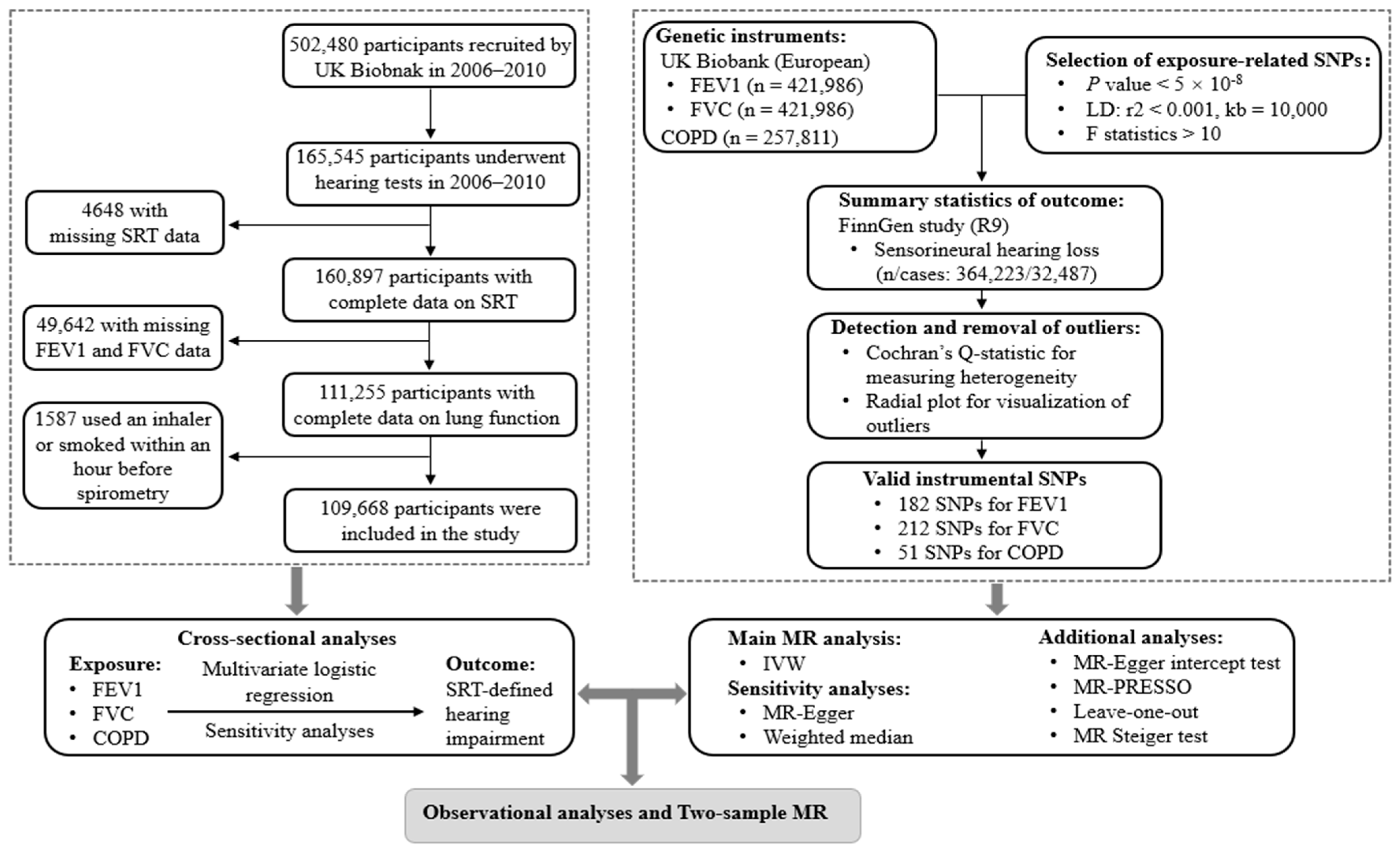
2. Materials and Methods
2.1. Study Design and Population
2.2. Spirometry and Definition of COPD
2.3. Definition of Hearing Impairment
2.4. Covariates
2.5. Data Sources and Genetic Instruments for MR Analyses
2.6. Statistical Analyses
2.6.1. Cross-Sectional Analyses
2.6.2. Two-Sample MR
3. Results
3.1. Associations of Lung Function and COPD with Hearing Impairment in UK Biobank
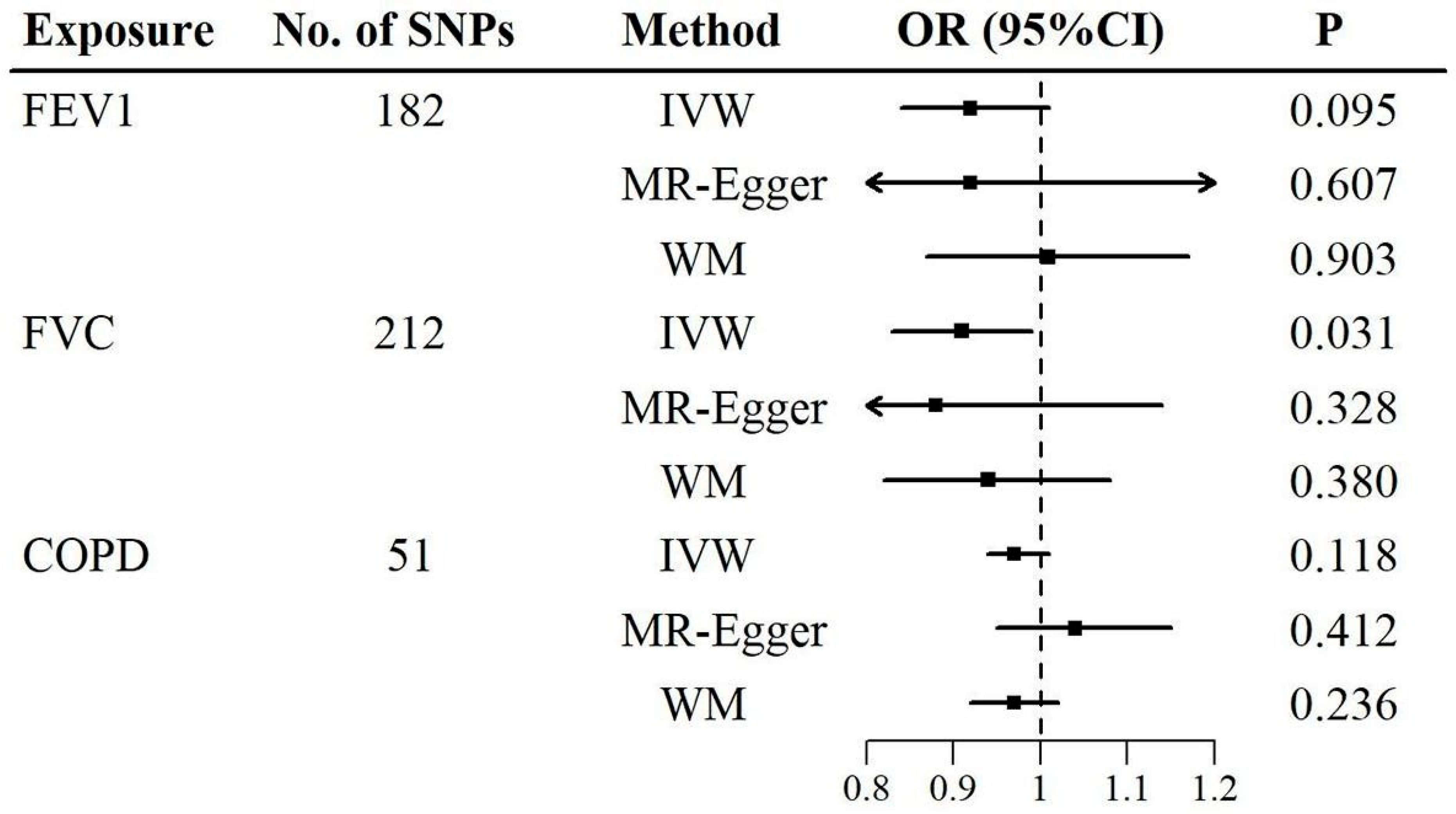
3.2. Results for MR Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nieman, C.L.; Oh, E.S. Hearing Loss. Ann. Intern. Med. 2020, 173, ITC81–ITC96. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Hearing. 2021. Available online: https://apps.who.int/iris/handle/10665/339913 (accessed on 11 June 2024).
- Collaborators, G.B.D.H.L. Hearing loss prevalence and years lived with disability, 1990–2019: Findings from the Global Burden of Disease Study 2019. Lancet 2021, 397, 996–1009. [Google Scholar] [CrossRef]
- Bayat, A.; Saki, N.; Nikakhlagh, S.; Mirmomeni, G.; Raji, H.; Soleimani, H.; Rahim, F. Is COPD associated with alterations in hearing? A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 14, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Chern, A.; Begasse de Dhaem, O.; Golub, J.S.; Lalwani, A.K. Chronic Obstructive Pulmonary Disease is a Risk Factor for Sensorineural Hearing Loss: A US Population Study. Otol. Neurotol. 2021, 42, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Aarhus, L.; Sand, M.; Engdahl, B. COPD and 20-year hearing decline: The HUNT cohort study. Respir. Med. 2023, 212, 107221. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Bush, A.; van den Berge, M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 2015, 385, 899–909. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhu, X.; London, S.J.; Sullivan, K.J.; Lutsey, P.L.; Windham, B.G.; Griswold, M.E.; Mosley, T.H., Jr. Association of Lung Function With Cognitive Decline and Incident Dementia in the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2023, 192, 1637–1646. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Köttgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. 2016, 27, 3253–3265. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Doiron, D.; de Hoogh, K.; Probst-Hensch, N.; Fortier, I.; Cai, Y.; De Matteis, S.; Hansell, A.L. Air pollution, lung function and COPD: Results from the population-based UK Biobank study. Eur. Respir. J. 2019, 54, 1802140. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Dawes, P.; Fortnum, H.; Moore, D.R.; Emsley, R.; Norman, P.; Cruickshanks, K.; Davis, A.; Edmondson-Jones, M.; McCormack, A.; Lutman, M. Hearing in middle age: A population snapshot of 40–69 year olds in the UK. Ear Hear. 2014, 35, e44–e51. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Spiller, W.; Del Greco, M.F.; Sheehan, N.; Thompson, J.; Minelli, C.; Davey Smith, G. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int. J. Epidemiol. 2018, 47, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, M.; Hao, K.; Bossé, Y.; Nickle, D.C.; Nie, Y.; Postma, D.S.; Laviolette, M.; Sandford, A.J.; Daley, D.D.; Hogg, J.C.; et al. Molecular mechanisms underlying variations in lung function: A systems genetics analysis. Lancet Respir. Med. 2015, 3, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Au Yeung, S.L.; Borges, M.C.; Lawlor, D.A. Association of Genetic Instrumental Variables for Lung Function on Coronary Artery Disease Risk: A 2-Sample Mendelian Randomization Study. Circ. Genom. Precis. Med. 2018, 11, e001952. [Google Scholar] [CrossRef]
- Nowak, C. Lung Function and Coronary Artery Disease Risk. Circ. Genom. Precis. Med. 2018, 11, e002137. [Google Scholar] [CrossRef]
- Godfrey, M.S.; Jankowich, M.D. The Vital Capacity Is Vital: Epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest 2016, 149, 238–251. [Google Scholar] [CrossRef]
- Agustí, A.; Noell, G.; Brugada, J.; Faner, R. Lung function in early adulthood and health in later life: A transgenerational cohort analysis. Lancet Respir. Med. 2017, 5, 935–945. [Google Scholar] [CrossRef]
- Guerra, S.; Carsin, A.E.; Keidel, D.; Sunyer, J.; Leynaert, B.; Janson, C.; Jarvis, D.; Stolz, D.; Rothe, T.; Pons, M.; et al. Health-related quality of life and risk factors associated with spirometric restriction. Eur. Respir. J. 2017, 49, 1602096. [Google Scholar] [CrossRef]
- Higbee, D.H.; Granell, R.; Sanderson, E.; Davey Smith, G.; Dodd, J.W. Lung function and cardiovascular disease: A two-sample Mendelian randomisation study. Eur. Respir. J. 2021, 58, 2003196. [Google Scholar] [CrossRef]
- Melén, E.; Faner, R.; Allinson, J.P.; Bui, D.; Bush, A.; Custovic, A.; Garcia-Aymerich, J.; Guerra, S.; Breyer-Kohansal, R.; Hallberg, J.; et al. Lung-function trajectories: Relevance and implementation in clinical practice. Lancet 2024, 403, 1494–1503. [Google Scholar] [CrossRef]
- Burney, P.G.; Hooper, R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax 2011, 66, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Portas, L.; Pereira, M.; Shaheen, S.O.; Wyss, A.B.; London, S.J.; Burney, P.G.J.; Hind, M.; Dean, C.H.; Minelli, C. Lung Development Genes and Adult Lung Function. Am. J. Respir. Crit. Care Med. 2020, 202, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Bui, D.S.; Walters, E.H.; Lowe, A.J.; Thompson, B.; Bowatte, G.; Thomas, P.; Garcia-Aymerich, J.; Jarvis, D.; Hamilton, G.S.; et al. Lifetime spirometry patterns of obstruction and restriction, and their risk factors and outcomes: A prospective cohort study. Lancet Respir. Med. 2023, 11, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Kwak, L.; Grams, M.E.; Yamagata, K.; Punjabi, N.M.; Kovesdy, C.P.; Coresh, J.; Matsushita, K. Lung Function and Incident Kidney Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. 2017, 70, 675–685. [Google Scholar] [CrossRef]
- Ramalho, S.H.R.; Shah, A.M. Lung function and cardiovascular disease: A link. Trends Cardiovasc. Med. 2021, 31, 93–98. [Google Scholar] [CrossRef]
- Qian, W.; Yang, L.; Li, T.; Li, W.; Zhou, J.; Xie, S. RNA modifications in pulmonary diseases. MedComm 2024, 5, e546. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Wong, E.; Yang, B.; Du, L.; Ho, W.H.; Lau, C.; Ke, Y.; Chan, Y.S.; Yung, W.H.; Wu, E.X. The multi-level impact of chronic intermittent hypoxia on central auditory processing. NeuroImage 2017, 156, 232–239. [Google Scholar] [CrossRef]
- Kociszewska, D.; Vlajkovic, S. Age-Related Hearing Loss: The Link between Inflammaging, Immunosenescence, and Gut Dysbiosis. Int. J. Mol. Sci. 2022, 23, 7348. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, J.; Liu, Q.; Miao, Z.; Chai, R.; Chen, W. Development of Chinese herbal medicine for sensorineural hearing loss. Acta Pharm. Sinica. B 2024, 14, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.; Shryane, N.; Kapadia, D.; Dawes, P.; Norman, P. Understanding ethnic inequalities in hearing health in the UK: A cross-sectional study of the link between language proficiency and performance on the Digit Triplet Test. BMJ Open 2020, 10, e042571. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’cOnnell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef]
- MRC IEU UK Biobank GWAS Pipeline, Version 2. Available online: https://data.bris.ac.uk/datasets/pnoat8cxo0u52p6ynfaekeigi/MRC%20IEU%20UK%20Biobank%20GWAS%20pipeline%20version%202.pdf (accessed on 18 January 2019).
- Sakornsakolpat, P.; Prokopenko, D.; Lamontagne, M.; Reeve, N.F.; Guyatt, A.L.; Jackson, V.E.; Shrine, N.; Qiao, D.; Bartz, T.M.; Kim, D.K.; et al. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat. Genet. 2019, 51, 494–505. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar]
- Guo, B.; Wang, C.; Zhu, Y.; Liu, Z.; Long, H.; Ruan, Z.; Lin, Z.; Fan, Z.; Li, Y.; Zhao, S. Causal associations of brain structure with bone mineral density: A large-scale genetic correlation study. Bone Res. 2023, 11, 37. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
| Hearing Impairment | p * | |||
|---|---|---|---|---|
| Total | No | Yes | ||
| No. of participants | 109,668 | 98,768 | 10,900 | |
| Age (years), median (IQR) | 58.00 (12.00) | 58.00 (13.00) | 63.00 (8.00) | <0.001 |
| FEV1 (liters), mean (SD) | 2.85 (0.75) | 2.88 (0.75) | 2.65 (0.71) | <0.001 |
| FVC (liters), mean (SD) | 3.76 (0.94) | 3.79 (0.94) | 3.54 (0.90) | <0.001 |
| Sex (%) | 0.022 | |||
| Male | 50,674 (46.2) | 45,524 (46.1) | 5150 (47.2) | |
| Female | 58,994 (53.8) | 53,244 (53.9) | 5750 (52.8) | |
| TDI (%) | <0.001 | |||
| 1st | 27,346 (24.9) | 24,883 (25.2) | 2463 (22.6) | |
| 2nd | 27,398 (25.0) | 24,682 (25.0) | 2716 (24.9) | |
| 3rd | 27,378 (25.0) | 24,749 (25.1) | 2629 (24.1) | |
| 4th | 27,375 (25.0) | 24,305 (24.6) | 3070 (28.2) | |
| Missing | 171 (0.2) | 149 (0.2) | 22 (0.2) | |
| Qualifications (%) | <0.001 | |||
| College or university degree | 37,694 (34.4) | 34,767 (35.2) | 2927 (26.9) | |
| A levels or AS levels | 13,058 (11.9) | 12,053 (12.2) | 1005 (9.2) | |
| or equivalent | ||||
| O levels, GCSEs, or CSEs | 30,350 (27.7) | 27,679 (28.0) | 2671 (24.5) | |
| or equivalent | ||||
| HND, HNC, NVQ, or other | 12,673 (11.6) | 11,168 (11.3) | 1505 (13.8) | |
| professional qualification | ||||
| None of the above | 15,141 (13.8) | 12,476 (12.6) | 2665 (24.4) | |
| Missing | 752 (0.7) | 625 (0.6) | 127 (1.2) | |
| Employment (%) | <0.001 | |||
| Employed | 62,668 (57.1) | 58,388 (59.1) | 4280 (39.3) | |
| Retired | 37,935 (34.6) | 32,236 (32.6) | 5699 (52.3) | |
| Other | 8743 (8.0) | 7855 (8.0) | 888 (8.1) | |
| Missing | 322 (0.3) | 289 (0.3) | 33 (0.3) | |
| Total | Hearing Impairment | p * | ||
|---|---|---|---|---|
| (n = 109,668) | No (n = 98,768) | Yes (n = 10,900) | ||
| Drink frequency (%) | <0.001 | |||
| Never | 6724 (6.1) | 5828 (5.9) | 896 (8.2) | |
| Special occasions only | 11,420 (10.4) | 9959 (10.1) | 1461 (13.4) | |
| One to three times a month | 12,277 (11.2) | 11,077 (11.2) | 1200 (11.0) | |
| Once or twice a week | 28,443 (25.9) | 25,680 (26.0) | 2763 (25.3) | |
| Three or four times a week | 26,572 (24.2) | 24,259 (24.6) | 2313 (21.2) | |
| Daily or almost daily | 24,177 (22.0) | 21,915 (22.2) | 2262 (20.8) | |
| Missing | 55 (0.1) | 50 (0.1) | 5 (0.0) | |
| Smoking (%) | <0.001 | |||
| Never | 58,985 (53.8) | 53,620 (54.3) | 5365 (49.2) | |
| Previous | 40,479 (36.9) | 36,051 (36.5) | 4428 (40.6) | |
| Current | 9890 (9.0) | 8838 (8.9) | 1052 (9.7) | |
| Missing | 314 (0.3) | 259 (0.3) | 55 (0.5) | |
| Body mass index, kg/m2 (%) | <0.001 | |||
| <25 | 36,671 (33.4) | 33,285 (33.7) | 3386 (31.1) | |
| ≥25 and <30 | 46,902 (42.8) | 42,283 (42.8) | 4619 (42.4) | |
| ≥30 | 26,008 (23.7) | 23,129 (23.4) | 2879 (26.4) | |
| Missing | 87 (0.1) | 71 (0.1) | 16 (0.1) | |
| Diabetes (%) | <0.001 | |||
| No | 104,431 (95.2) | 94,317 (95.5) | 10,114 (92.8) | |
| Yes | 5046 (4.6) | 4285 (4.3) | 761 (7.0) | |
| Missing | 191 (0.2) | 166 (0.2) | 25 (0.2) | |
| Cardiovascular diseases (%) | <0.001 | |||
| None | 78,963 (72.0) | 71,921 (72.8) | 7042 (64.6) | |
| Hypertension | 23,176 (21.1) | 20,413 (20.7) | 2763 (25.3) | |
| Heart attack, angina, | 2479 (2.3) | 2092 (2.1) | 387 (3.6) | |
| or stroke | ||||
| Hypertension, and heart | 2469 (2.3) | 2069 (2.1) | 400 (3.7) | |
| attack, angina, or stroke | ||||
| Missing | 2581 (2.4) | 2273 (2.3) | 308 (2.8) | |
| Work noise exposure (%) | <0.001 | |||
| No | 84,034 (76.6) | 76,390 (77.3) | 7644 (70.1) | |
| Yes | 24,779 (22.6) | 21,618 (21.9) | 3161 (29.0) | |
| Missing | 855 (0.8) | 760 (0.8) | 95 (0.9) | |
| Music noise exposure (%) | <0.001 | |||
| No | 94,773 (86.4) | 85,158 (86.2) | 9615 (88.2) | |
| Yes | 13,475 (12.3) | 12,350 (12.5) | 1125 (10.3) | |
| Missing | 1420 (1.3) | 1260 (1.3) | 160 (1.5) | |
| COPD | <0.001 | |||
| No | 101,332 (92.4) | 91,383 (92.5) | 9949 (91.3) | |
| Yes | 8336 (7.6) | 7385 (7.5) | 951 (8.7) | |
| Exposure | Unadjusted OR (95% CI) | p | Adjusted * OR (95% CI) | p |
|---|---|---|---|---|
| FEV1, per IQR | 0.64 (0.62, 0.65) | <0.001 | 0.80 (0.77, 0.84) | <0.001 |
| FVC, per IQR | 0.67 (0.65, 0.69) | <0.001 | 0.80 (0.76, 0.83) | <0.001 |
| COPD (FEV1/FVC < LLN) † | 1.18 (1.10, 1.27) | <0.001 | 1.10 (1.02, 1.18) | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Cui, F.; Yin, G.; Shi, M.; Aximu, N.; Tian, Y.; Sun, Y. Role of Lung Function, Chronic Obstructive Pulmonary Disease on Hearing Impairment: Evidence for Causal Effects and Clinical Implications. Audiol. Res. 2025, 15, 88. https://doi.org/10.3390/audiolres15040088
Yuan L, Cui F, Yin G, Shi M, Aximu N, Tian Y, Sun Y. Role of Lung Function, Chronic Obstructive Pulmonary Disease on Hearing Impairment: Evidence for Causal Effects and Clinical Implications. Audiology Research. 2025; 15(4):88. https://doi.org/10.3390/audiolres15040088
Chicago/Turabian StyleYuan, Lanlai, Feipeng Cui, Ge Yin, Mengwen Shi, Nadida Aximu, Yaohua Tian, and Yu Sun. 2025. "Role of Lung Function, Chronic Obstructive Pulmonary Disease on Hearing Impairment: Evidence for Causal Effects and Clinical Implications" Audiology Research 15, no. 4: 88. https://doi.org/10.3390/audiolres15040088
APA StyleYuan, L., Cui, F., Yin, G., Shi, M., Aximu, N., Tian, Y., & Sun, Y. (2025). Role of Lung Function, Chronic Obstructive Pulmonary Disease on Hearing Impairment: Evidence for Causal Effects and Clinical Implications. Audiology Research, 15(4), 88. https://doi.org/10.3390/audiolres15040088








