African Mole-Rats May Have High Bone Conduction Sensitivity to Counterbalance Low Air Conduction Sensitivity
Abstract
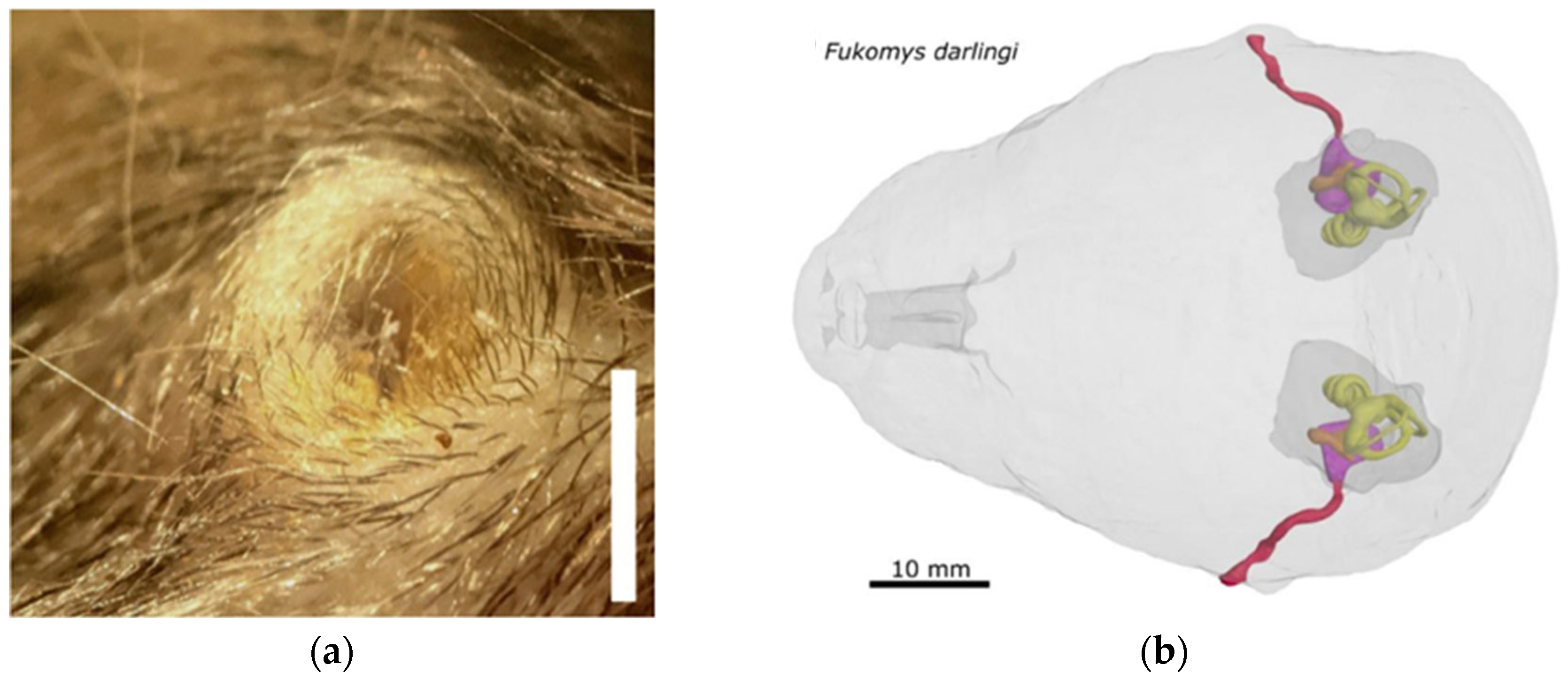
1. Introduction
2. Air Conduction vs. Bone Conduction
3. Other Methodological Problems
4. Resolving a Long-Standing Issue
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pyott, S.J.; van Tuinen, M.; Screven, L.A.; Schrode, K.M.; Bai, J.-P.; Barone, C.M.; Price, S.D.; Lysakowski, A.; Sanderford, M.; Kumar, S.; et al. Functional, morphological, and evolutionary characterization of hearing in subterranean, eusocial African mole-rats. Curr. Biol. 2020, 30, 4329–4341. [Google Scholar] [CrossRef] [PubMed]
- Okanoya, K.; Yosida, S.; Barone, C.M.; Applegate, D.T.; Brittan-Powell, E.F.; Dooling, R.J.; Park, T.J. Auditory-vocal coupling in the naked mole-rat, a mammal with poor auditory thresholds. J. Comp. Physiol. A 2018, 204, 905–914. [Google Scholar] [CrossRef]
- Dent, M.L.; Screven, L.A.; Kobrina, A. Hearing in rodents. In Rodent Bioacoustics; Dent, M.L., Fay, R.R., Popper, A.N., Eds.; Springer: Cham, Switzerland, 2018; pp. 71–105. [Google Scholar] [CrossRef]
- Begall, S.; Burda, H.; Caspar, K.R. Fukomys anselli (Rodentia: Bathyergidae). Mamm. Species 2021, 53, 160–173. [Google Scholar] [CrossRef]
- Heffner, R.S.; Heffner, H.E. Degenerate hearing and sound localization in naked mole rats (Heterocephalus glaber), with an overview of central auditory structures. J. Comp. Neurol. 1993, 331, 418–433. [Google Scholar] [CrossRef]
- Lange, S.; Burda, H.; Wegner, R.E.; Dammann, P.; Begall, S.; Kawalika, M. Living in a “stethoscope”: Burrow-acoustics promote auditory specializations in subterranean rodents. Naturwissenschaften 2007, 94, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Buffenstein, R.; Amoroso, V.; Andziak, B.; Avdieiev, S.; Azpurua, J.; Barker, A.J.; Bennett, N.C.; Brieño-Enríquez, M.A.; Bronner, G.N.; Coen, C.; et al. The naked truth: A comprehensive clarification and classification of current ‘myths’ in naked mole-rat biology. Biol. Rev. Camb. Philos. Soc. 2021, 97, 115–140. [Google Scholar] [CrossRef]
- Manley, G.A.; Maat, B.; Begall, S.; Malkemper, P.; Caspar, K.R.; Moritz, L.; van Dijk, P. Otoacoustic emissions in African mole-rats. Hear. Res. 2024, 445, 108994. [Google Scholar] [CrossRef] [PubMed]
- Caspar, K.R.; Heinrich, A.; Mellinghaus, L.; Gerhardt, P.; Begall, S. Evoked auditory potentials from African mole-rats and coruros reveal disparity in subterranean rodent hearing. J. Exp. Biol. 2021, 224, jeb243371. [Google Scholar] [CrossRef]
- Gerhardt, P.; Henning, Y.; Begall, S.; Malkemper, E.P. Audiograms of three subterranean rodent species (genus Fukomys) determined by auditory brainstem responses reveal extremely poor high-frequency hearing. J. Exp. Biol. 2017, 220, 4377–4382. [Google Scholar] [CrossRef][Green Version]
- Bruns, V.; Müller, M.; Hofer, W.; Heth, G.; Nevo, E. Inner ear structure and electrophysiological audiograms of the subterranean mole rat, Spalax ehrenbergi. Hear. Res. 1988, 33, 1–9. [Google Scholar] [CrossRef]
- Christensen, C.B.; Christensen-Dalsgaard, J.; Brandt, C.; Madsen, P.T. Hearing with an atympanic ear: Good vibration and poor sound-pressure detection in the royal python, Python regius. J. Exp. Biol. 2012, 215, 331–342. [Google Scholar] [CrossRef]
- Burda, H.; Bruns, V.; Hickman, G.C. The ear in subterranean Insectivora and Rodentia in comparison with ground-dwelling representatives. I. Sound conducting system of the middle ear. J. Morphol. 1992, 214, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Vice, E.N.; Lagestee, S.; Browe, B.M.; Deb, D.; Smith, E.S.J.; Park, T.J. Sensory systems of the African naked mole-rat. In The Extraordinary Biology of the Naked Mole-Rat; Buffenstein, R., Park, T.J., Holmes, M.M., Eds.; Springer: Cham, Switzerland, 2021; pp. 137–156. [Google Scholar] [CrossRef]
- Mason, M.J.; Cornwall, H.L.; Smith, E.S.J. Ear structures of the naked mole-rat, Heterocephalus glaber, and its relatives (Rodentia: Bathyergidae). PLoS ONE 2016, 11, e0167079. [Google Scholar] [CrossRef] [PubMed]
- Ketten, D.R. Structure and function in whale ears. Bioacoustics 1997, 8, 103–135. [Google Scholar] [CrossRef]
- Tonndorf, J. Bone conduction. In Foundations of Modern Auditory Theory; Tobias, J.V., Ed.; Academic Press: New York, NY, USA, 1972; pp. 195–237. [Google Scholar]
- Ketten, D.R. Cetacean ears. In Hearing by Whales and Dolphins; Au, W.W.L., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 2000; pp. 43–108. [Google Scholar] [CrossRef]
- Sohmer, H. Soft tissue conduction: Review, mechanisms, and implications. Trends Hear. 2017, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sohmer, H. Soft tissue conduction is the third mode of auditory stimulation. Auris Nasus Larynx 2020, 47, 168–169. [Google Scholar] [CrossRef]
- Bell, A. The remarkable frog ear: Implications for vertebrate hearing. J. Hear. Sci. 2016, 6, 17–30. [Google Scholar] [CrossRef]
- Narins, P.M.; Feng, A.S.; Fay, R.R.; Popper, A.N. Hearing and Sound Communication in Amphibians; Springer: New York, USA, 2006. [Google Scholar] [CrossRef]
- Popper, A.N.; Fay, R.R. Rethinking sound detection by fishes. Hear. Res. 2011, 273, 25–36. [Google Scholar] [CrossRef]
- Barker, A.J.; Koch, U.; Lewin, G.R.; Pyott, S.J. Hearing and vocalizations in the naked mole-rat. In The Extraordinary Biology of the Naked Mole-Rat; Buffenstein, R., Park, T.J., Holmes, M.M., Eds.; Springer: Cham, Switzerland, 2021; pp. 157–195. [Google Scholar] [CrossRef]
- Békésy, G.V. Experiments in Hearing; Wever, E.G., Ed.; McGraw-Hill: New York, NY, USA, 1960. [Google Scholar]
- Mason, M.J.; Lai, F.W.; Li, J.; Nevo, E. Middle ear structure and bone conduction in Spalax, Eospalax, and Tachyorytes mole-rats (Rodentia: Spalacidae). J. Morphol. 2010, 271, 462–472. [Google Scholar] [CrossRef]
- Kimchi, T.; Reshef, M.; Terkel, J. Evidence for the use of reflected self-generated seismic waves for spatial orientation in a blind subterranean mammal. J. Comput. Biol. 2005, 208, 647–659. [Google Scholar] [CrossRef]
- Rado, R.; Himelfarb, M.; Arensburg, B.; Terkel, J.; Wollberg, Z. Are seismic communication signals transmitted by bone conduction in the blind mole rat? Hear. Res. 1989, 41, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Heth, G.; Frankenberg, E.; Raz, A.; Nevo, E. Vibrational communication in subterranean mole rats (Spalax ehrenbergi). Behav. Ecol. Sociobiol. 1987, 21, 31–33. [Google Scholar] [CrossRef]
- Heth, G.; Frankenberg, E.; Pratt, H.; Nevo, E. Seismic communication in the blind subterranean mole-rat: Patterns of head thumping and of their detection in the Spalax ehrenbergi superspecies in Israel. J. Zool. 1991, 224, 633–638. [Google Scholar] [CrossRef]
- Mason, M.J. Structure and function of the mammalian middle ear. I: Large middle ears in small desert mammals. J. Anat. 2016, 228, 284–299. [Google Scholar] [CrossRef]
- Narins, P.M. Vibration communication in vertebrates. In Ecology of Sensing; Barth, F.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 127–148. [Google Scholar]
- Narins, P.M.; Willi, U.B. The golden mole middle ear: A sensor for airborne and substrate-borne vibrations. In Frontiers in Sensing; Barth, F.G., Ed.; Springer: Vienna, Austria, 2012; pp. 275–286. [Google Scholar] [CrossRef]
- Mason, M.J.; Narins, P.M. Seismic sensitivity in the desert golden mole (Eremitalpa granti): A review. J. Comp. Psychol. 2002, 116, 158–163. [Google Scholar] [CrossRef]
- Narins, P.M.; Lewis, E.R.; Jarvis, J.J.; O’riain, J. The use of seismic signals by fossorial southern African mammals: A neuroethological gold mine. Brain Res. Bull. 1997, 44, 641–646. [Google Scholar] [CrossRef]
- Ellsperman, S.E.; Nairn, E.M.; Stucken, E.Z. Review of bone conduction hearing devices. Audiol. Res. 2021, 11, 207–219. [Google Scholar] [CrossRef]
- Narins, P.M.; Lewis, E.R. The vertebrate ear as an exquisite seismic sensor. J. Acoust. Soc. Am. 1984, 76, 1384–1387. [Google Scholar] [CrossRef]
- Heffner, R.S.; Heffner, H.E. Hearing and sound localization in blind mole rats (Spalax ehrenbergi). Hear. Res. 1992, 62, 206–216. [Google Scholar] [CrossRef]
- Kössl, M.; Frank, G.; Burda, H.; Müller, M. Acoustic distortion products from the cochlea of the blind African mole rat, Cryptomys spec. J. Comp. Physiol. A 1996, 178, 427–434. [Google Scholar] [CrossRef]
- Rado, R.; Terkel, J.; Wollberg, Z. Seismic communication signals in the blind mole-rat (Spalax ehrinbergi): Electrophysiological and behavioral evidence for their processing by the auditory system. J. Comp. Physiol. A 1998, 183, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Wollberg, Z.; Rado, R.; Terkel, J. Seismic communication signals in the blind mole rat are processed by the auditory system. In Acoustical Signal Processing in the Central Auditory System; Syka, J., Ed.; Springer: Boston, MA, USA, 1997; pp. 469–476. [Google Scholar] [CrossRef]
- Nevo, E.; Heth, G.; Pratt, H. Seismic communication in a blind subterranean mammal: A major somatosensory mechanism in adaptive evolution underground. Proc. Nat. Acad. Sci. USA 1991, 88, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.J.; Narins, P.M. Seismic sensitivity and communication in subterranean mammals. In The Use of Vibrations in Communication: Properties, Mechanisms and Function Across Taxa; O’Connell-Rodwell, C.E., Ed.; Transworld Research Network: Kerala, India, 2010; pp. 1–19. [Google Scholar]
- Christensen-Dalsgaard, J.; Manley, G.A. The malleable middle ear: An underappreciated player in the evolution of hearing in vertebrates. In Insights from Comparative Hearing Research; Köppl, C., Manley, G.A., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 2014; pp. 157–191. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, A. African Mole-Rats May Have High Bone Conduction Sensitivity to Counterbalance Low Air Conduction Sensitivity. Audiol. Res. 2025, 15, 64. https://doi.org/10.3390/audiolres15030064
Bell A. African Mole-Rats May Have High Bone Conduction Sensitivity to Counterbalance Low Air Conduction Sensitivity. Audiology Research. 2025; 15(3):64. https://doi.org/10.3390/audiolres15030064
Chicago/Turabian StyleBell, Andrew. 2025. "African Mole-Rats May Have High Bone Conduction Sensitivity to Counterbalance Low Air Conduction Sensitivity" Audiology Research 15, no. 3: 64. https://doi.org/10.3390/audiolres15030064
APA StyleBell, A. (2025). African Mole-Rats May Have High Bone Conduction Sensitivity to Counterbalance Low Air Conduction Sensitivity. Audiology Research, 15(3), 64. https://doi.org/10.3390/audiolres15030064





