Auditory Perception Outcomes in Children with Deafness and Additional Disabilities 12 Months After Cochlear Implant Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Eligibility Criteria
- -
- A confirmed diagnosis of severe or profound bilateral sensorineural hearing loss.
- -
- At least one additional disability affecting neurodevelopment and/or a syndromic diagnosis associated with HL.
- -
- Anatomically intact auditory nerves and a cochlea without malformations, confirmed via imaging.
- -
- Receipt of a unilateral cochlear implant through the public health system.
- -
- Completion of auditory speech perception assessments both pre-implantation and 12 months post-activation.
- -
- Consistent use of the cochlear implant sound processor.
- -
- No postsurgical complications requiring reimplantation.
2.3. Demographics
2.4. Speech Perception Testing Procedures
2.5. Statistical Analysis
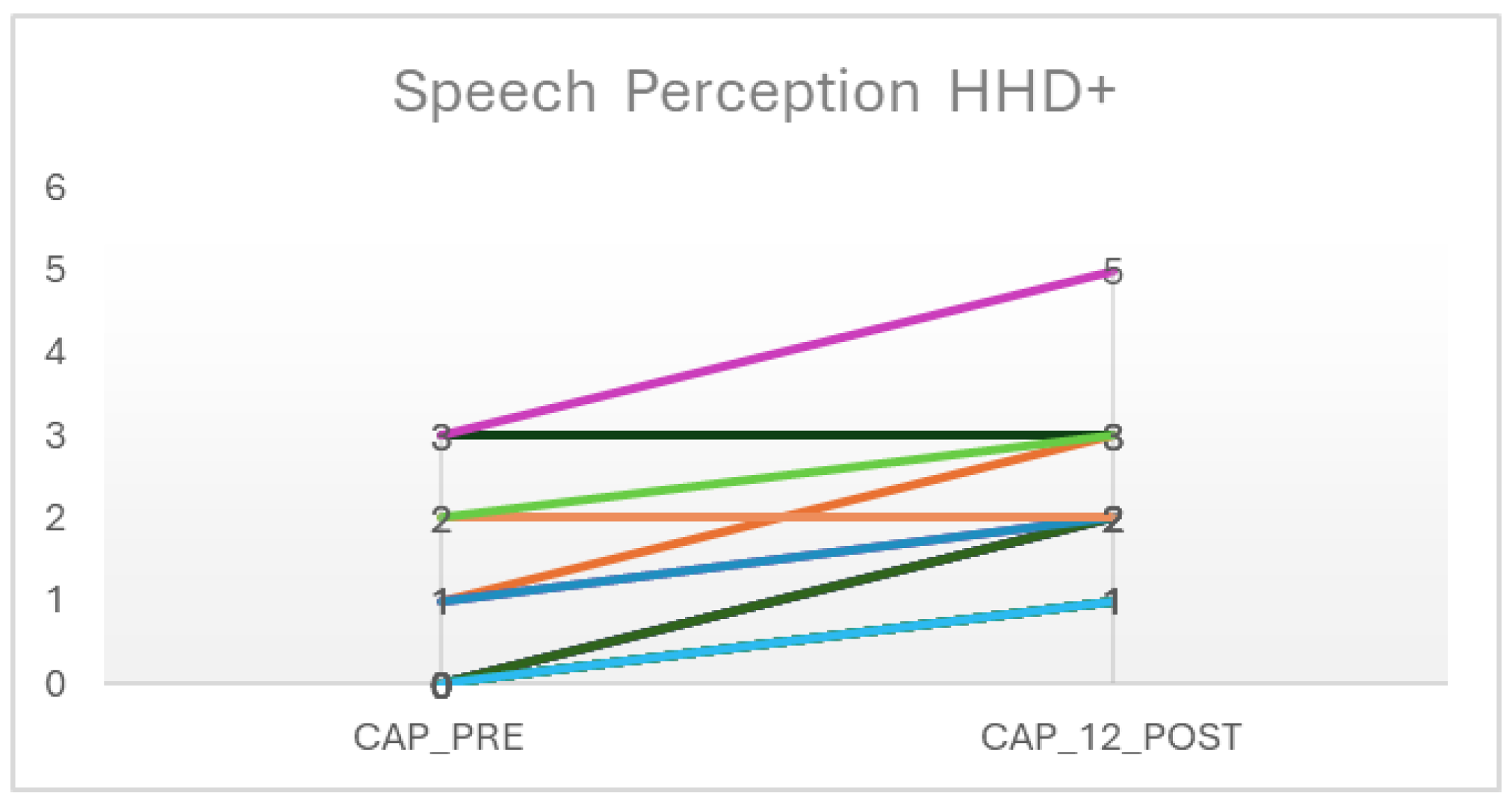
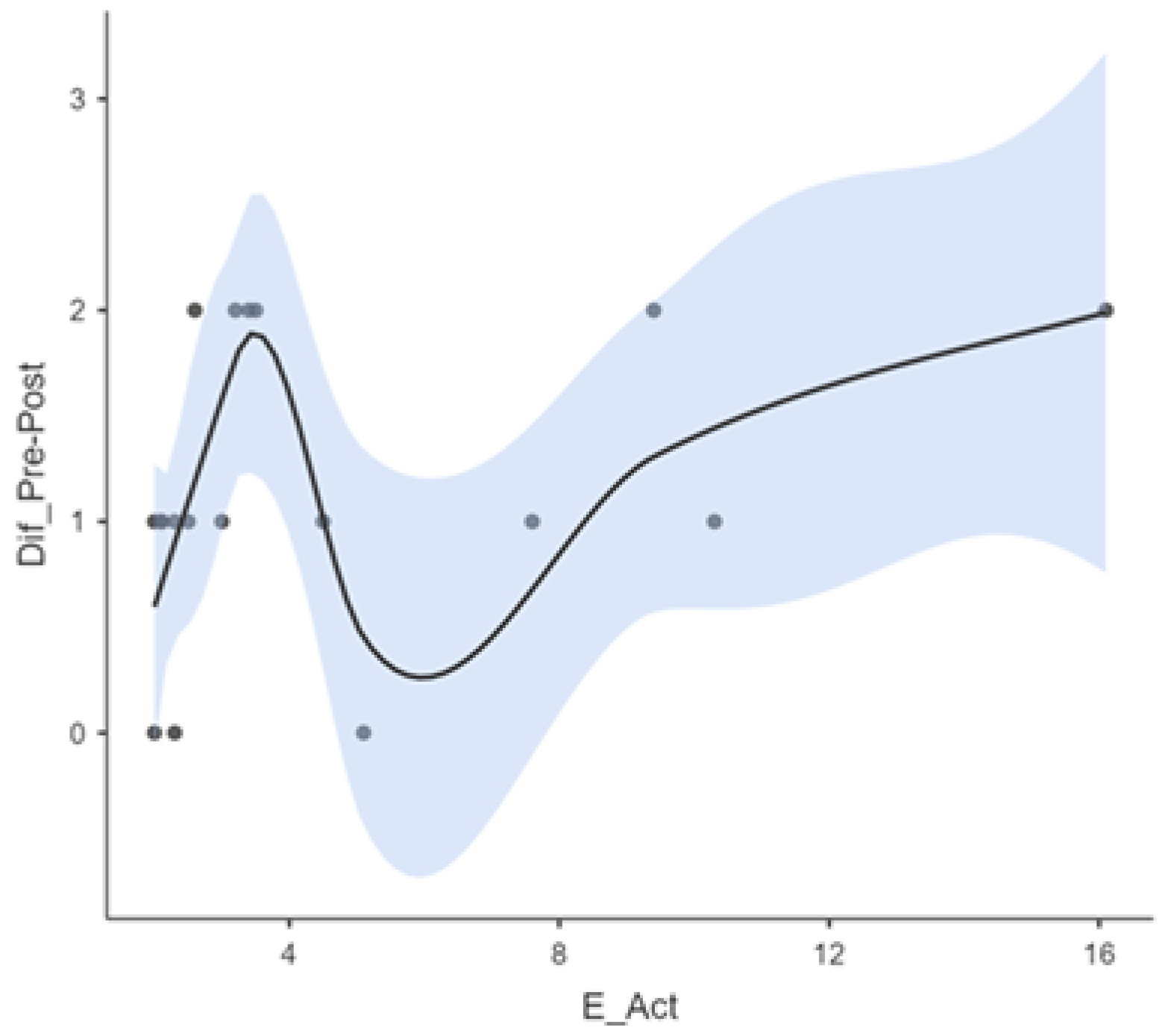
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism spectrum disorder |
| CAP | Category of Auditory Performance |
| CI | Cochlear implant |
| HL/D | Hearing loss or deafness |
References
- World Health Organization. World Report on Hearing; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Yoshinaga-Itano, C.; Manchaiah, V.; Hunnicutt, C. Outcomes of Universal Newborn Screening Programs: Systematic Review. J. Clin. Med. 2021, 10, 2784. [Google Scholar] [CrossRef] [PubMed]
- Edmond, K.; Chadha, S.; Hunnicutt, C.; Strobel, N.; Manchaiah, V.; Yoshinga-Itano, C. Effectiveness of universal newborn hearing screening: A systematic review and meta-analysis. J. Glob. Health 2022, 12, 12006. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Infant Hearing. Year 2019 JCIH Position Statement.pdf. 2019. Available online: https://digitalcommons.usu.edu/jehdi/vol4/iss2/1/ (accessed on 20 February 2025).
- Núñez-Batalla, F.; Jáudenes-Casaubón, C.; Sequí-Canet, J.M.; Vivanco-Allende, A.; Zubicaray-Ugarteche, J. Sordera infantil con discapacidad asociada (DA+): Recomendaciones CODEPEH. Acta Otorrinolaringol. Esp. 2021, 74, 386–396. [Google Scholar] [CrossRef]
- Wakil, N.; Fitzpatrick, E.M.; Olds, J.; Schramm, D.; Whittingham, J. Long-term outcome after cochlear implantation in children with additional developmental disabilities. Int. J. Audiol. 2014, 53, 587–594. [Google Scholar] [CrossRef]
- Hayward, D.V.; Ritter, K.; Grueber, J.; Howarth, T. Outcomes That Matter for Children with Severe Multiple Disabilities who use Cochlear Implants: The First Step in an Instrument Development Process. Can. J. Speech Lang. Pathol. Audiol. 2013, 37, 58–68. [Google Scholar]
- Cejas, I.; Hoffman, M.; Quittner, A. Outcomes and benefits of pediatric cochlear implantation in children with additional disabilities: A review and report of family influences on outcomes. Pediatr. Health Med. Ther. 2015, 6, 45–63. [Google Scholar] [CrossRef]
- Ching, T.Y.; Dillon, H.; Marnane, V.; Hou, S.; Day, J.; Seeto, M.; Crowe, K.; Street, L.; Thomson, J.; Van Buynder, P.; et al. Outcomes of Early- and Late-Identified Children at 3 Years of Age: Findings From a Prospective Population-Based Study. Ear Hear. 2013, 34, 535–552. [Google Scholar] [CrossRef]
- Ministerio de Salud. Guía Clínica Auge Tratamiento de la Hipoacusia Moderada en Menores de 2 Años; Ministerio de Salud: Santiago, Chile, 2013.
- Ministerio de Salud. Guía de Práctica Clínica Implante Coclear. Rehabilitación de Personas en Situación de Discapacidad por Hipoacusia Sensorioneural Severa a Profunda Bilateral; Ministerio de Salud: Santiago, Chile, 2008.
- Ministerio de Salud. Hipoacusia Neurosensorial Bilateral del Prematutro; Series Guías Cínicas Minsal; Ministerio de Salud: Santiago, Chile, 2009.
- Minsal. Resumen Ejecutivo. Guía de Práctica Clínica Hipoacusia en Recién Nacidos, Niños y Niñas Menores de 4 Años. 2018. Available online: https://docs.bvsalud.org/biblioref/2024/02/967241/hipoacusia-neurosensorial-bilateral-del-prematuro-guias-clinica_o6oqB6h.pdf (accessed on 1 February 2025).
- Rawes, C.; Ngaage, L.M.; Mackenzie, R.; Martin, J.; Cordingley, A.; Raine, C. A review of the outcomes of children with designated additional needs receiving cochlear implantation for severe to profound hearing loss. Cochlear Implant. Int. 2021, 22, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wiley, S.; John, R.S.; Lindow-Davies, C. Children Who Are Deaf or Hard of Hearing PLUS. 2022. Available online: https://www.infanthearing.org/ehdi-ebook/ (accessed on 28 January 2025).
- Chapman, D.A.; Stampfel, C.C.; Bodurtha, J.N.; Dodson, K.M.; Pandya, A.; Lynch, K.B.; Kirby, R.S. Impact of Co-Occurring Birth Defects on the Timing of Newborn Hearing Screening and Diagnosis. Am. J. Audiol. 2011, 20, 132–139. [Google Scholar] [CrossRef]
- Mortari Moret, A. Pruebas y Protocolos de Evaluación en Español y Portugués. In La Práctica Auditivo Verbal (PAV) en Latinoamérica. Intervención en la Población Infantil con Pérdida Auditiva y sus Familias; RehAB Latin American Leaders Committee, Ed.; Advanced Bionics Latin America: São Paulo, Brazil, 2023; pp. 137–148. [Google Scholar]
- Archbold, S.; Lutman, M.E.; Nikolopoulos, T. Categories of Auditory Performance. Ann. Otol. Rhinol. Laryngol. 1995, 166, 312–314. [Google Scholar]
- Albalawi, Y.; Nidami, M.; Almohawas, F.; Hagr, A.; Garadat, S.N. Categories of Auditory Performance and Speech Intelligibility Ratings in Prelingually Deaf Children with Bilateral Implantation. Am. J. Audiol. 2019, 28, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Archbold, S.; Nikolopoulos, T.P.; O’Donoghue, G.M.; Lutman, M.E. Educational placement of deaf children following cochlear implantation. Br. J. Audiol. 1998, 32, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Jalil-Abkenar, S.S.; Ashori, M.; Pourmohamadreza-Tajrishi, M.; Hasanzadeh, S. Auditory Perception and Verbal Intelligibility in Children with Cochlear Implant, Hearing Aids and Normal Hearing. Pract. Clin. Psychol. 2013, 1, 141–147. [Google Scholar]
- Rafferty, A.; Martin, J.; Strachan, D.; Raine, C. Cochlear implantation in children with complex needs—Outcomes. Cochlear Implants Int. 2013, 14, 61–66. [Google Scholar] [CrossRef]
- Davidson, L.S. La Audición Acústica Temprana y el Papel de la Percepción Segmental y Suprasegmental del Habla en el Lenguaje Hablado y la Alfabetización; Washington University School of Medicine: Washington, DC, USA, 2020. [Google Scholar]
- Van de Velde, D.J.; Schiller, N.O.; Levelt, C.C.; Van Heuven, V.J.; Beers, M.; Briaire, J.J.; Frijns, J.H. Prosody perception and production by children with cochlear implants. J. Child Lang. 2019, 46, 111–141. [Google Scholar] [CrossRef]
- Magalhães, A.; Samuel, P.; Goffi-Gomez, M.; Tsuji, R.; Brito, R.; Bento, R. Audiological outcomes of cochlear implantation in Waardenburg Syndrome. Int. Arch. Otorhinolaryngol. 2014, 17, 285–290. [Google Scholar] [CrossRef][Green Version]
- Kral, A.; Sharma, A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012, 35, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Lachowska, M.; Pastuszka, A.; Łukaszewicz-Moszyńska, Z.; Mikołajewska, L.; Niemczyk, K. Cochlear implantation in autistic children with profound sensorineural hearing loss. Braz. J. Otorhinolaryngol. 2018, 84, 15–19. [Google Scholar] [CrossRef]
- Ching, T.Y.C.C.; Dillon, H.; Leigh, G.; Cupples, L. Learning from the Longitudinal Outcomes of Children with Hearing Impairment (LOCHI) study: Summary of 5-year findings and implications. Int. J. Audiol. 2018, 57, S105–S111. [Google Scholar] [CrossRef]
- Dettman, S.J.; Dowell, R.C.; Choo, D.; Arnott, W.; Abrahams, Y.; Davis, A.; Dornan, D.; Leigh, J.; Constantinescu, G.; Cowan, R.; et al. Long-term Communication Outcomes for Children Receiving Cochlear Implants Younger Than 12 Months. Otol. Neurotol. 2016, 37, e82–e95. [Google Scholar] [CrossRef]
- Zimmerman-Phillips, S.; Robbins, A.M.; Osberger, M.J. Assessing Cochlear Implant Benefit in Very Young Children. Ann. Otol. Rhinol. Laryngol. 2000, 109 (Suppl. 12), 42–43. [Google Scholar] [CrossRef] [PubMed]
- Obrycka, A.; Lorens, A.; García, J.L.P.; Piotrowska, A.; Skarzynski, H. Validation of the LittlEARS Auditory Questionnaire in cochlear implanted infants and toddlers. Int. J. Pediatr. Otorhinolaryngol. 2017, 93, 107–116. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S. The Ling Sound Test: What Is Its Relevance in the New Zealand Classroom? Kairaranga 2014, 15, 48–55. [Google Scholar] [CrossRef]
- Jatana, K.R.; Thomas, D.; Weber, L.; Mets, M.B.; Silverman, J.B.; Young, N.M. Usher Syndrome: Characteristics and Outcomes of Pediatric Cochlear Implant Recipients. Otol. Neurotol. 2013, 34, 484–489. [Google Scholar] [CrossRef]
- Young, N.M.; Weil, C.; Tournis, E. Redefining Cochlear Implant Benefits to Appropriately Include Children with Additional Disabilities. In Pediatric Cochlear Implantation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 213–224. [Google Scholar] [CrossRef]
| 0 | No awareness of environmental sounds |
| 1 | Awareness of environmental sounds |
| 2 | Response to speech sounds |
| 3 | Identification of environmental sounds |
| 4 | Discrimination of some speech sounds without lip reading |
| 5 | Comprehension of common sentences without lip reading |
| 6 | Conversation comprehension without lip reading |
| 7 | Use of the telephone with a known speaker |
| Additional Disabilities | Frequency | % of Total | % Accumulated |
|---|---|---|---|
| Global developmental delay | 2 | 11.1% | 11.1% |
| Autism spectrum disorder, cleft of soft and hard palates | 1 | 5.6% | 16.7% |
| Waardenburg syndrome | 1 | 5.6% | 22.2% |
| Autism spectrum disorder | 1 | 5.6% | 27.8% |
| Prematurity, microcephaly, hypothyroidism | 1 | 5.6% | 33.3% |
| Cerebral palsy (spastic tetraparesis), prematurity, hydrocephalus, global developmental delay, epilepsy | 1 | 5.6% | 38.9% |
| Waardenburg syndrome, mild intellectual disability | 1 | 5.6% | 44.4% |
| Global developmental delay, hypotony, autism spectrum disorder | 1 | 5.6% | 50.0% |
| Prematurity, cytomegalovirus, retinitis | 1 | 5.6% | 55.6% |
| Prematurity, global developmental delay, learning disability | 1 | 5.6% | 61.1% |
| Severe spinal cord aplasia, learning disability | 1 | 5.6% | 66.7% |
| Global developmental delay, learning disability | 1 | 5.6% | 72.2% |
| Extrapyramidal cerebral palsy, global developmental delay, microcephaly, prematurity | 1 | 5.6% | 77.8% |
| Prematurity, mixed cerebral palsy | 1 | 5.6% | 83.3% |
| Prematurity, global developmental delay, strabismus | 1 | 5.6% | 88.9% |
| Prematurity, severe hemolytic disease due to Rh- mother, retinopathy | 1 | 5.6% | 94.4% |
| Usher syndrome, corneal dystrophy | 1 | 5.6% | 100.0% |
| A_DETEC | A_Act | CAP_Preimp | CAP_Post | Dif_Pre-Post | |
|---|---|---|---|---|---|
| N | 16 | 18 | 18 | 18 | 18 |
| Lost | 2 | 0 | 0 | 0 | 0 |
| Average | 1.61 | 4.67 | 0.722 | 1.94 | 1.17 |
| Median | 0.950 | 3.10 | 0.00 | 2.00 | 1.00 |
| Standard Deviation | 1.53 | 3.83 | 1.07 | 1.06 | 0.707 |
| Minimum | 0.110 | 2.00 | 0 | 1 | 0 |
| Maximum | 5.00 | 16.1 | 3 | 5 | 2 |
| Shapiro–Wilk W | 0.782 | 0.721 | 0.706 | 0.796 | 0.807 |
| Shapiro–Wilk p-value | 0.002 | <0.001 | <0.001 | 0.001 | 0.002 |
| Statistic | p | Mean Difference | EE of the Difference | Effect Size | ||||
|---|---|---|---|---|---|---|---|---|
| CAP_Preimp | CAP_Post | W of Wilcoxon | 0.00 a | <0.001 | −1.50 | 0.152 | Biserial correlation of ranges | −1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Pantanalli, C.; Bravo-Torres, S. Auditory Perception Outcomes in Children with Deafness and Additional Disabilities 12 Months After Cochlear Implant Activation. Audiol. Res. 2025, 15, 47. https://doi.org/10.3390/audiolres15030047
Martínez-Pantanalli C, Bravo-Torres S. Auditory Perception Outcomes in Children with Deafness and Additional Disabilities 12 Months After Cochlear Implant Activation. Audiology Research. 2025; 15(3):47. https://doi.org/10.3390/audiolres15030047
Chicago/Turabian StyleMartínez-Pantanalli, Celia, and Sofía Bravo-Torres. 2025. "Auditory Perception Outcomes in Children with Deafness and Additional Disabilities 12 Months After Cochlear Implant Activation" Audiology Research 15, no. 3: 47. https://doi.org/10.3390/audiolres15030047
APA StyleMartínez-Pantanalli, C., & Bravo-Torres, S. (2025). Auditory Perception Outcomes in Children with Deafness and Additional Disabilities 12 Months After Cochlear Implant Activation. Audiology Research, 15(3), 47. https://doi.org/10.3390/audiolres15030047






