Defining Potential Pathomechanisms Behind an Impaired Canal Function at the Video-Head Impulse Test in Canal Dehiscence. Reply to Ionescu et al. Comment on “Castellucci et al. Impaired Vestibulo-Ocular Reflex on Video Head Impulse Test in Superior Canal Dehiscence: “Spontaneous Plugging” or Endolymphatic Flow Dissipation? Audiol. Res. 2023, 13, 802–820”
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABG | Air-bon gap |
| AC | Air-conduction |
| EH | Endolymphatic hydrops |
| HRCT | High resolution CT |
| HSC | Horizontal semicircular canal |
| LARP | Left anterior—right posterior |
| MD | Meniere’s disease |
| MRI | Magnetic resonance imaging |
| PSC | Posterior semicircular canal |
| RALP | Right anterior—left posterior |
| SCD | Superior canal dehiscence |
| SSC | Superior semicircular canal |
| SVINT | Skull vibration-induced nystagmus test |
| TWS | Third window syndrome |
| VEMPs | Vestibular-evoked myogenic potentials |
| vHIT | Video-head impulse test |
| VOR | Vestibulo-ocular reflex |
References
- Ionescu, E.C.; Mustea, E.; Reynard, P.; Thai-Van, H. Comment on Castellucci et al. Impaired Vestibulo-Ocular Reflex on Video Head Impulse Test in Superior Canal Dehiscence: “Spontaneous Plugging” or Endolymphatic Flow Dissipation? Audiol. Res. 2023, 13, 802–820. Audiol. Res. 2024, 14, 857–860. [Google Scholar] [CrossRef]
- Zhou, G.; Gopen, Q.; Poe, D.S. Clinical and Diagnostic Characterization of Canal Dehiscence Syndrome: A Great Otologic Mimicker. Otol. Neurotol. 2007, 28, 920–926. [Google Scholar] [CrossRef]
- Ward, B.K.; Carey, J.P.; Minor, L.B. Superior Canal Dehiscence Syndrome: Lessons from the First 20 Years. Front. Neurol. 2017, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Albernaz, P.L.M.; Cusin, F.S.; Ramos, B.F.; Cal, R.; Maia, F.C.Z.E. Vestibular Disorders Limited to the Vertical Semicircular Canals. Int. Arch. Otorhinolaryngol. 2024, 28, e587–e591. [Google Scholar] [CrossRef]
- Tarnutzer, A.A.; Bockisch, C.J.; Buffone, E.; Weber, K.P. Association of posterior semicircular canal hypofunction on video-head-impulse testing with other vestibulo-cochlear deficits. Clin. Neurophysiol. 2017, 128, 1532–1541. [Google Scholar] [CrossRef]
- Castellucci, A.; Piras, G.; Del Vecchio, V.; Ferri, G.G.; Ghidini, A.; Brandolini, C. Which Inner Ear Disorders Lie Behind a Selective Posterior Semicircular Canal Hypofunction on Video Head Impulse Test? Otol. Neurotol. 2021, 42, 573–584. [Google Scholar] [CrossRef]
- Allum, J.H.J.; Honegger, F. Correlations Between Multi-plane vHIT Responses and Balance Control After Onset of an Acute Unilateral Peripheral Vestibular Deficit. Otol. Neurotol. 2020, 41, e952–e960. [Google Scholar] [CrossRef] [PubMed]
- Allum, J.H.J.; Honegger, F. Improvement of Asymmetric Vestibulo-Ocular Reflex Responses Following Onset of Vestibular Neuritis Is Similar Across Canal Planes. Front. Neurol. 2020, 11, 565125. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, X.; Zhang, Y.; Liu, D. A follow-Up Study with the Video Head Impulse Test for the patients with vestibular neuritis. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020, 34, 990–992+998. [Google Scholar] [CrossRef]
- Psillas, G.; Petrou, I.; Printza, A.; Sfakianaki, I.; Binos, P.; Anastasiadou, S.; Constantinidis, J. Video Head Impulse Test (vHIT): Value of Gain and Refixation Saccades in Unilateral Vestibular Neuritis. J. Clin. Med. 2022, 11, 3467. [Google Scholar] [CrossRef]
- Castellucci, A.; Brandolini, C.; Piras, G.; Del Vecchio, V.; Modugno, G.C.; Ghidini, A.; Pirodda, A.A. Spontaneous plugging of superior canal: Two possible natural evolutions of an “unstable” dehiscence. J. Vestib. Res. 2018, 28, 1–281. [Google Scholar]
- Castellucci, A.; Piras, G.; Del Vecchio, V.; Crocetta, F.M.; Maiolo, V.; Ferri, G.G.; Ghidini, A.; Brandolini, C. The effect of superior canal dehiscence size and location on audiometric measurements, vestibular-evoked myogenic potentials and video-head impulse testing. Eur. Arch. Otorhinolaryngol. 2021, 278, 997–1015. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, A.; Martellucci, S.; Malara, P.; Botti, C.; Del Vecchio, V.; Brandolini, C.; Ferri, G.G.; Ghidini, A.; Armato, E. Possible pathomechanisms accounting for both sound/pressure-induced eye movements and video head impulse test data in superior canal dehiscence. Acta Otolaryngol. 2021, 141, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, A.; Malara, P.; Martellucci, S.; Alfarghal, M.; Brandolini, C.; Piras, G.; Armato, E.; Ruberto, R.R.; Brizzi, P.; Presutti, L.; et al. Impaired Vestibulo-Ocular Reflex on Video Head Impulse Test in Superior Canal Dehiscence: “Spontaneous Plugging” or Endolymphatic Flow Dissipation? Audiol. Res. 2023, 13, 802–820. [Google Scholar] [CrossRef]
- Castellucci, A.; Dumas, G.; Abuzaid, S.M.; Armato, E.; Martellucci, S.; Malara, P.; Alfarghal, M.; Ruberto, R.R.; Brizzi, P.; Ghidini, A.; et al. Posterior Semicircular Canal Dehiscence with Vestibulo-Ocular Reflex Reduction for the Affected Canal at the Video-Head Impulse Test: Considerations to Pathomechanisms. Audiol. Res. 2024, 14, 317–332. [Google Scholar] [CrossRef]
- Rabbitt, R.D.; Boyle, R.; Highstein, S.M. Physiology of the semicircular canals after surgical plugging. Ann. N. Y. Acad. Sci. 2001, 942, 274–286. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Bae, Y.J.; Kim, M.; Song, J.-J.; Choi, B.Y.; Koo, J.-W. Changes in Vestibulo-Ocular Reflex Gain After Surgical Plugging of Superior Semicircular Canal Dehiscence. Front. Neurol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Stultiens, J.J.A.; Guinand, N.; Van Rompaey, V.; Fornos, A.P.; Kunst, H.P.M.; Kingma, H.; van de Berg, R. The resilience of the inner ear-vestibular and audiometric impact of transmastoid semicircular canal plugging. J. Neurol. 2022, 269, 5229–5238. [Google Scholar] [CrossRef]
- Renteria, A.E.; Elblidi, A.; Altamami, N.; Alhabib, S.; Saliba, I. Video Head Impulse Test Demonstrates a Residual Function after Plugging of Dehiscent Superior Semicircular Canal. Otol Neurotol. 2023, 44, 252–259. [Google Scholar] [CrossRef]
- Matiño-Soler, E.; Esteller-More, E.; Martin-Sanchez, J.-C.; Perez-Fernandez, N. Normative data on angular vestibulo-ocular responses in the yaw axis measured using the video head impulse test. Otol. Neurotol. 2015, 36, 466–471. [Google Scholar] [CrossRef]
- Kim, T.S.; Lim, H.W.; Yang, C.J.; Kim, Y.H.; Choi, W.R.; Kim, Y.R.; Park, J.W.; Kang, B.C.; Park, H.J. Changes of video head impulse test results in lateral semicircular canal plane by different peak head velocities in patients with vestibular neuritis. Acta Otolaryngol. 2018, 138, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Baek, W.; Jae Lee, Y.; Oh, J.; Cho, S.I.; Nam, G.S. Assessing the Vestibulo-ocular Reflex of Contralesional Sides According to Head Impulse Velocity Utilizing the Video Head Impulse Test in Patients with Vestibular Neuritis. J. Int. Adv. Otol. 2024, 20, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Halmagyi, G.M.; Chen, L.; MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Curthoys, I.S. The Video Head Impulse Test. Front Neurol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Yun, J.M.; Kim, S.H.; Bae, S.H. Vestibular dysfunction in lateral semicircular canal dysplasia. Front. Neurol. 2024, 15, 1341812. [Google Scholar] [CrossRef] [PubMed]
- Iversen, M.M.; Rabbitt, R.D. Wave Mechanics of the Vestibular Semicircular Canals. Biophys. J. 2017, 113, 1133–1149. [Google Scholar] [CrossRef]
- Iversen, M.M.; Rabbitt, R.D. Biomechanics of Third Window Syndrome. Front. Neurol. 2020, 11, 891. [Google Scholar] [CrossRef]
- Constant Ionescu, E.; Idriss, S.; Reynard, P.; Ltaief-Boudrigua, A.; Thai-Van, H. Persistent Positional Vertigo in a Patient with Partial “Auto-Plugged” Superior Semicircular Canal Dehiscence: A Case Study. J. Int. Adv. Otol. 2022, 18, 188–191. [Google Scholar] [CrossRef]
- Carey, J.P.; Hirvonen, T.P.; Hullar, T.E.; Minor, L.B. Acoustic responses of vestibular afferents in a model of superior canal dehiscence. Otol. Neurotol. 2004, 25, 345–352. [Google Scholar] [CrossRef]
- Curthoys, I.S. The new vestibular stimuli: Sound and vibration-anatomical, physiological and clinical evidence. Exp. Brain. Res. 2017, 235, 957–972. [Google Scholar] [CrossRef]
- Dlugaiczyk, J.; Burgess, A.M.; Goonetilleke, S.C.; Sokolic, L.; Curthoys, I.S. Superior Canal Dehiscence Syndrome: Relating Clinical Findings With Vestibular Neural Responses From a Guinea Pig Model. Otol. Neurotol. 2019, 40, e406–e414. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Smith, C.M.; Burgess, A.M.; Dlugaiczyk, J. A Review of Neural Data and Modelling to Explain How a Semicircular Canal Dehiscence (SCD) Causes Enhanced VEMPs, Skull Vibration Induced Nystagmus (SVIN), and the Tullio Phenomenon. Audiol. Res. 2023, 13, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Burgess, A.M.; McGarvie, L.A.; Curthoys, I.S. An indicator of probable semicircular canal dehiscence: Ocular vestibular evoked myogenic potentials to high frequencies. Otolaryngol. Head. Neck. Surg. 2013, 149, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Burgess, A.M.; Manzari, L.; Pastras, C.J. A Single Fast Test for Semicircular Canal Dehiscence-oVEMP n10 to 4000 Hz-Depends on Stimulus Rise Time. Audiol. Res. 2022, 12, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Dumas, G.; Curthoys, I.; Castellucci, A.; Dumas, L.; Peultier-Celli, L.; Armato, E.; Malara, P.; Perrin, P.; Schmerber, S. Skull Vibration-Induced Nystagmus in Superior Semicircular Canal Dehiscence: A New Insight into Vestibular Exploration-A Review. Audiol. Res. 2024, 14, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Cremer, P.D.; Minor, L.B.; Carey, J.P.; Della Santina, C.C. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology 2000, 55, 1833–1841. [Google Scholar] [CrossRef]
- Guerra Jiménez, G.; Pérez Fernández, N. Reduction in posterior semicircular canal gain by age in video head impulse testing. Observational study. Acta Otorrinolaringol. Esp. 2016, 67, 15–22. [Google Scholar] [CrossRef]

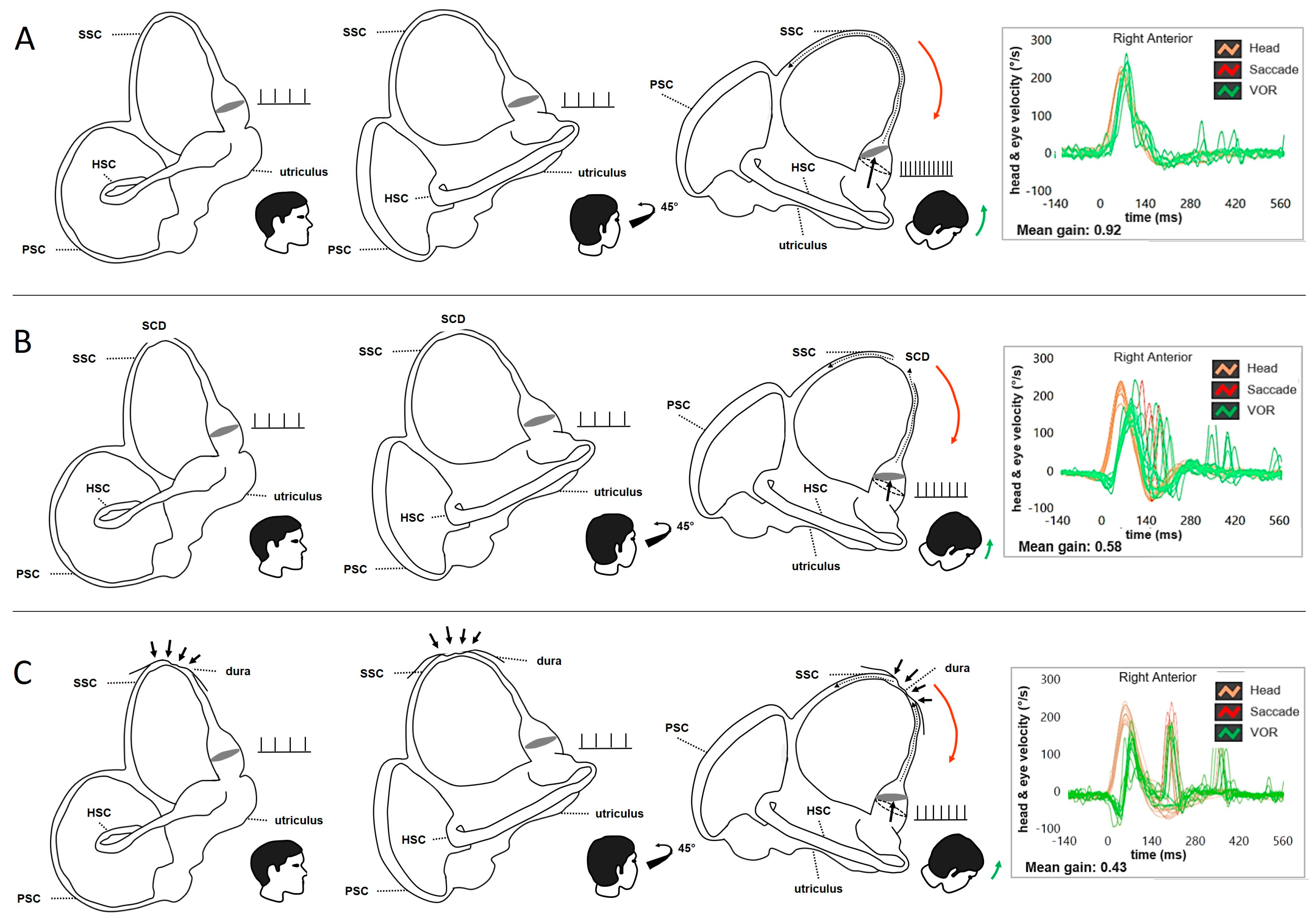
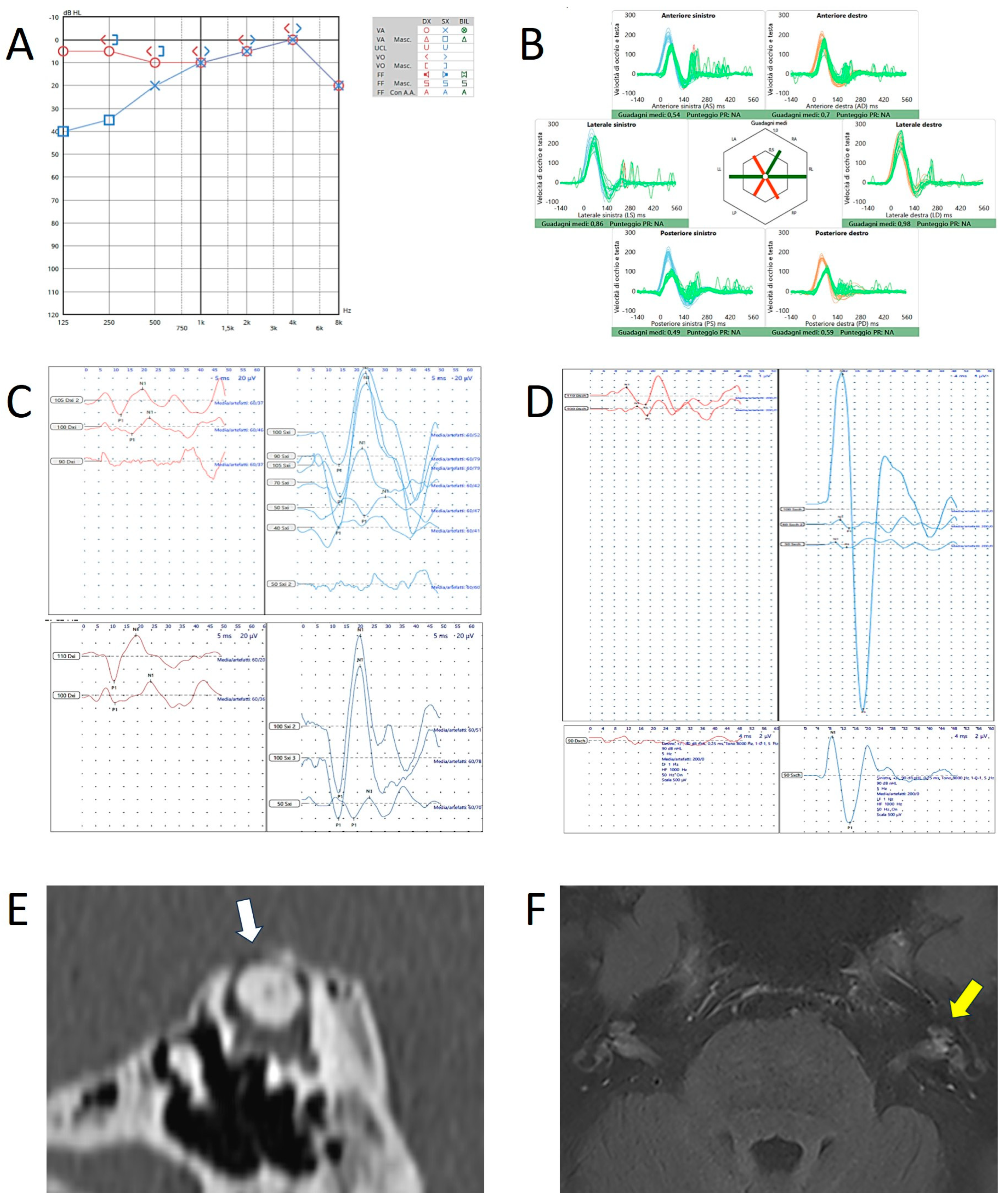
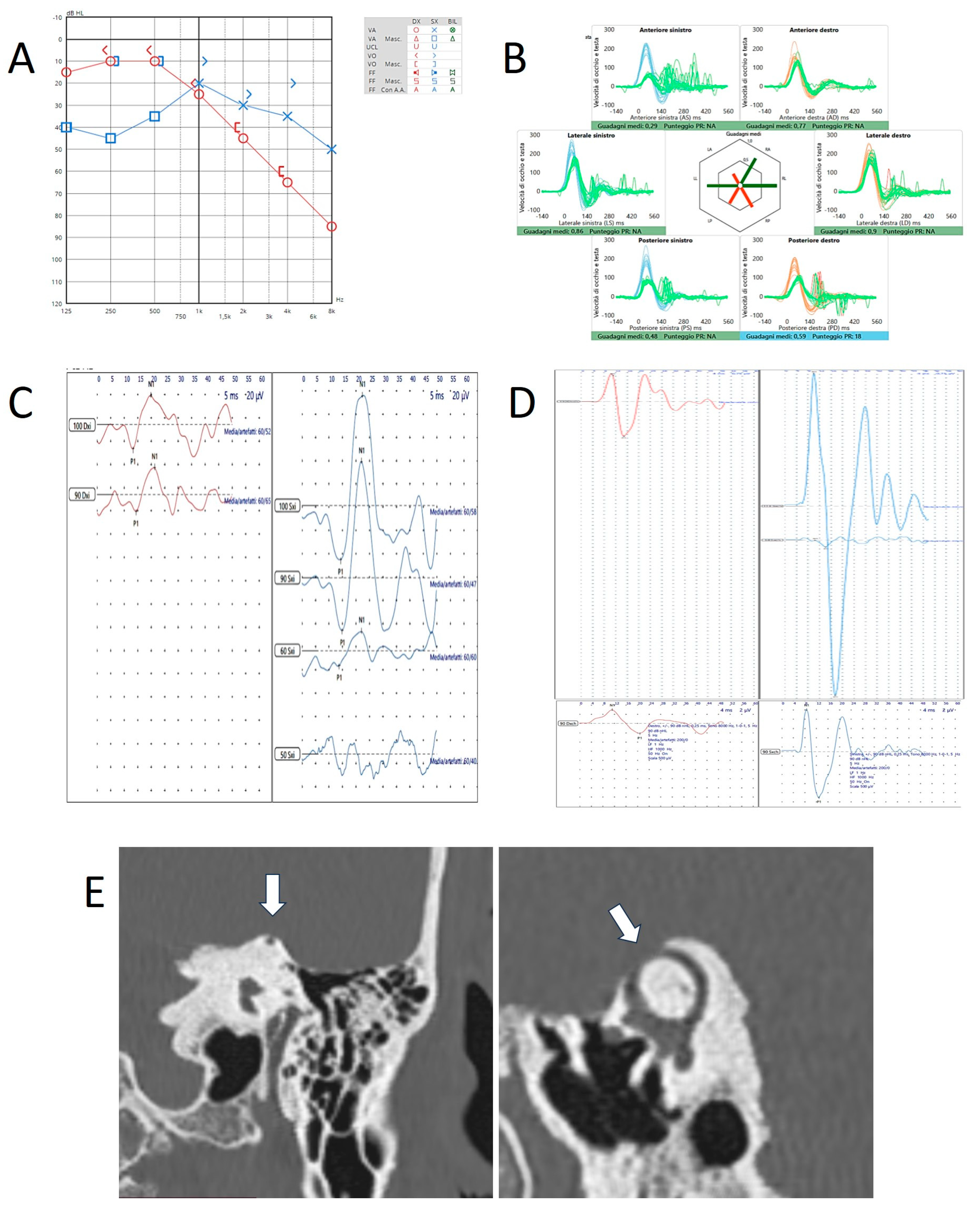
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malara, P.; Martellucci, S.; Castellucci, A. Defining Potential Pathomechanisms Behind an Impaired Canal Function at the Video-Head Impulse Test in Canal Dehiscence. Reply to Ionescu et al. Comment on “Castellucci et al. Impaired Vestibulo-Ocular Reflex on Video Head Impulse Test in Superior Canal Dehiscence: “Spontaneous Plugging” or Endolymphatic Flow Dissipation? Audiol. Res. 2023, 13, 802–820”. Audiol. Res. 2025, 15, 32. https://doi.org/10.3390/audiolres15020032
Malara P, Martellucci S, Castellucci A. Defining Potential Pathomechanisms Behind an Impaired Canal Function at the Video-Head Impulse Test in Canal Dehiscence. Reply to Ionescu et al. Comment on “Castellucci et al. Impaired Vestibulo-Ocular Reflex on Video Head Impulse Test in Superior Canal Dehiscence: “Spontaneous Plugging” or Endolymphatic Flow Dissipation? Audiol. Res. 2023, 13, 802–820”. Audiology Research. 2025; 15(2):32. https://doi.org/10.3390/audiolres15020032
Chicago/Turabian StyleMalara, Pasquale, Salvatore Martellucci, and Andrea Castellucci. 2025. "Defining Potential Pathomechanisms Behind an Impaired Canal Function at the Video-Head Impulse Test in Canal Dehiscence. Reply to Ionescu et al. Comment on “Castellucci et al. Impaired Vestibulo-Ocular Reflex on Video Head Impulse Test in Superior Canal Dehiscence: “Spontaneous Plugging” or Endolymphatic Flow Dissipation? Audiol. Res. 2023, 13, 802–820”" Audiology Research 15, no. 2: 32. https://doi.org/10.3390/audiolres15020032
APA StyleMalara, P., Martellucci, S., & Castellucci, A. (2025). Defining Potential Pathomechanisms Behind an Impaired Canal Function at the Video-Head Impulse Test in Canal Dehiscence. Reply to Ionescu et al. Comment on “Castellucci et al. Impaired Vestibulo-Ocular Reflex on Video Head Impulse Test in Superior Canal Dehiscence: “Spontaneous Plugging” or Endolymphatic Flow Dissipation? Audiol. Res. 2023, 13, 802–820”. Audiology Research, 15(2), 32. https://doi.org/10.3390/audiolres15020032







