Exploring Electrode Placements to Optimize the Identification and Measurement of Early Auditory Evoked Potentials
Abstract
:1. Introduction
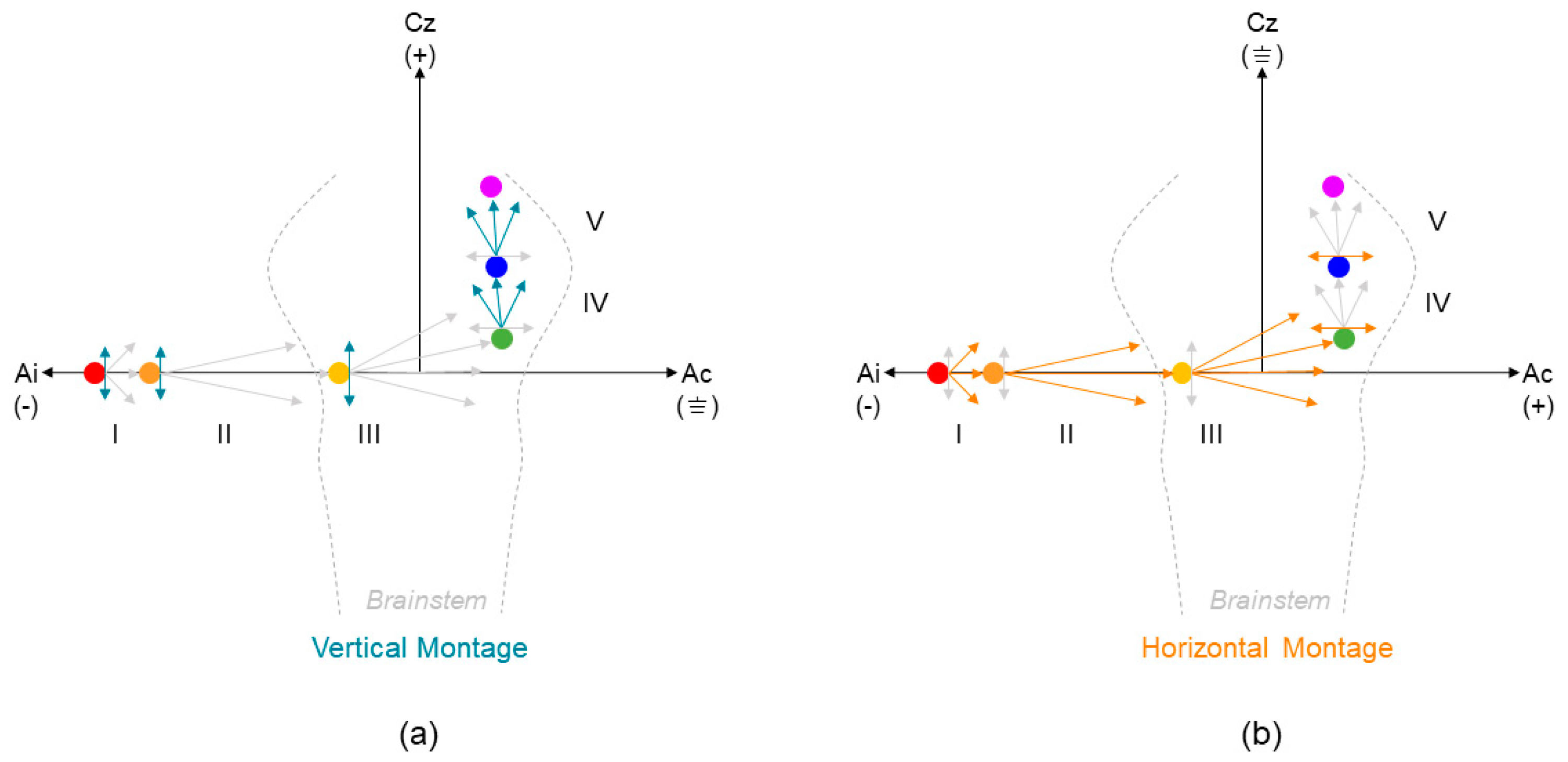
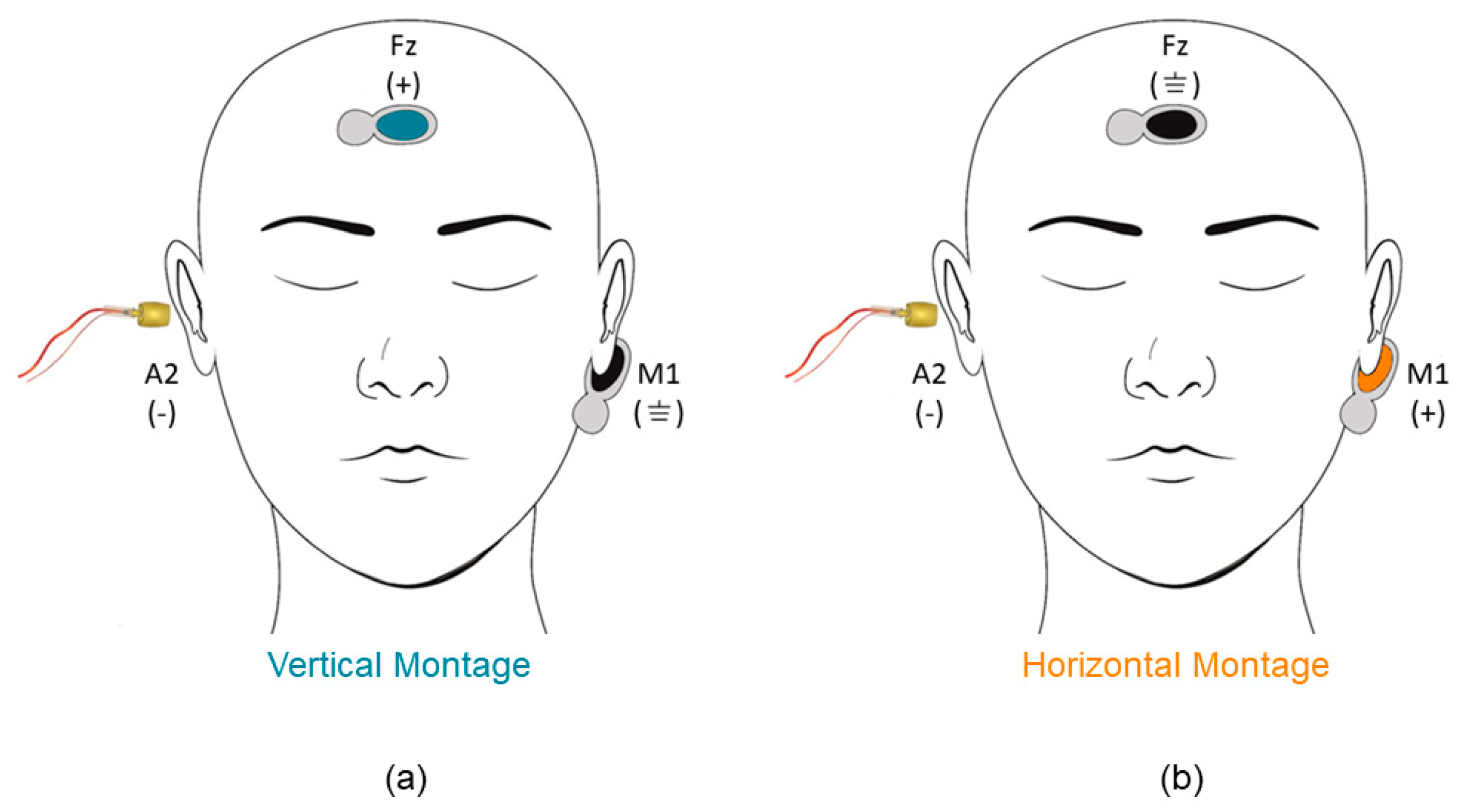
2. Materials and Methods
3. Results
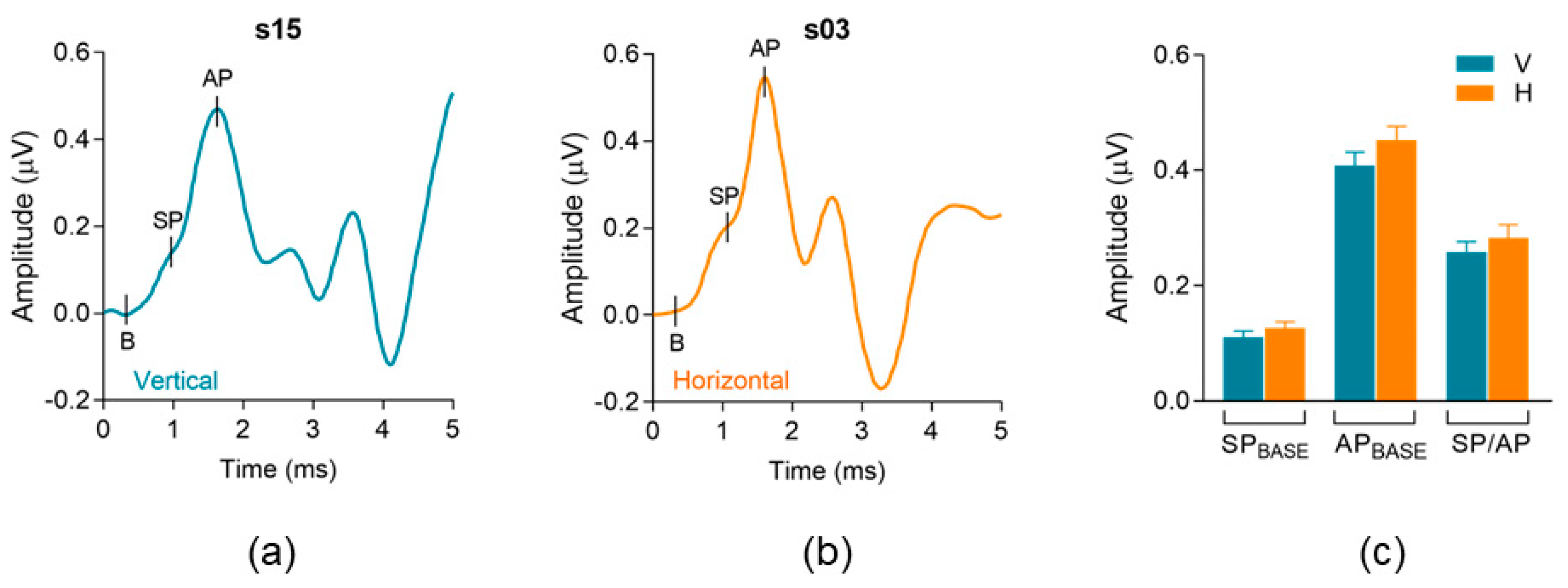
3.1. Electrode Montage Effects on Standard Measures of Electrocochleography (ECochG)
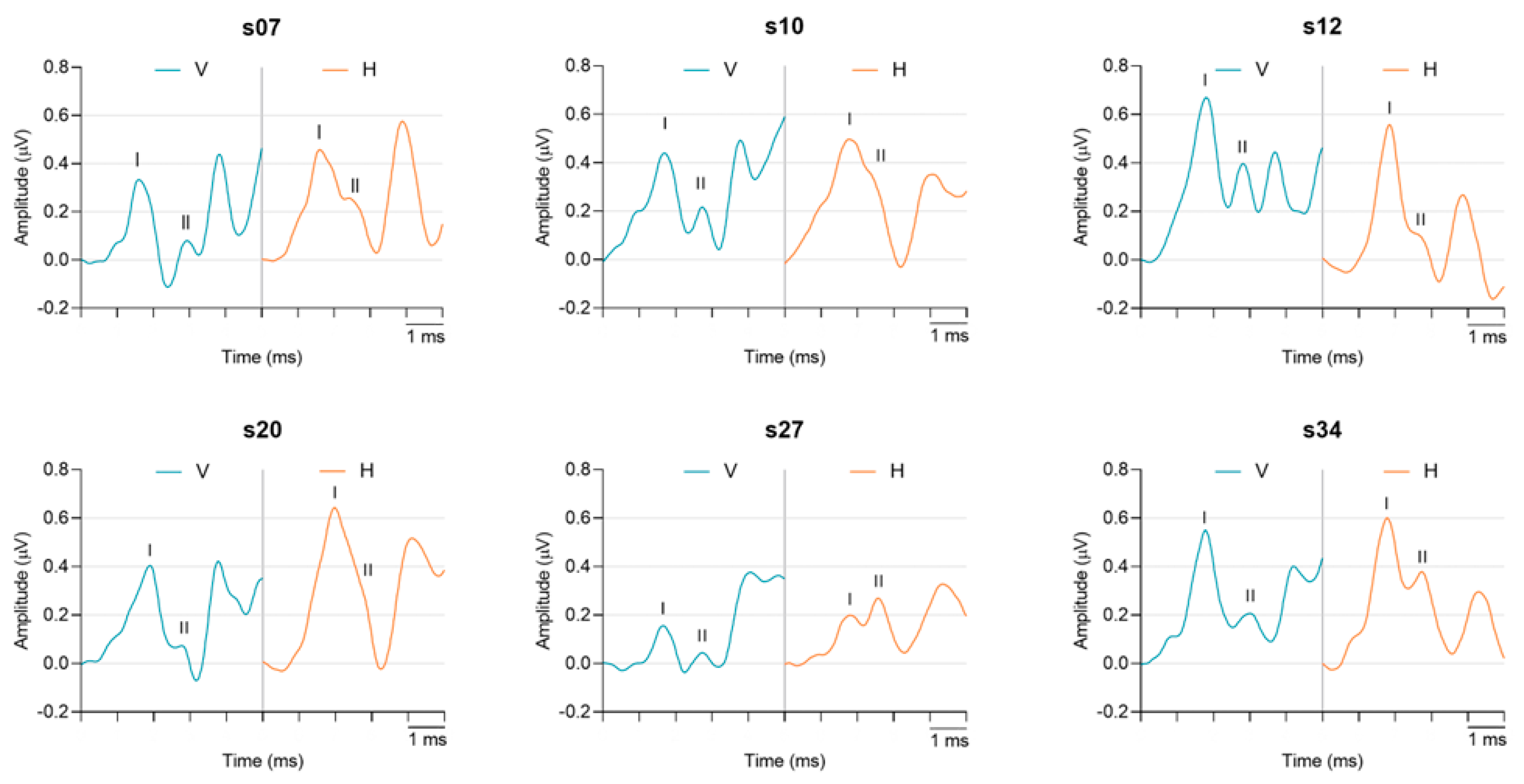
3.2. Electrode Montage Effects on Measures of Early Auditory Evoked Activity
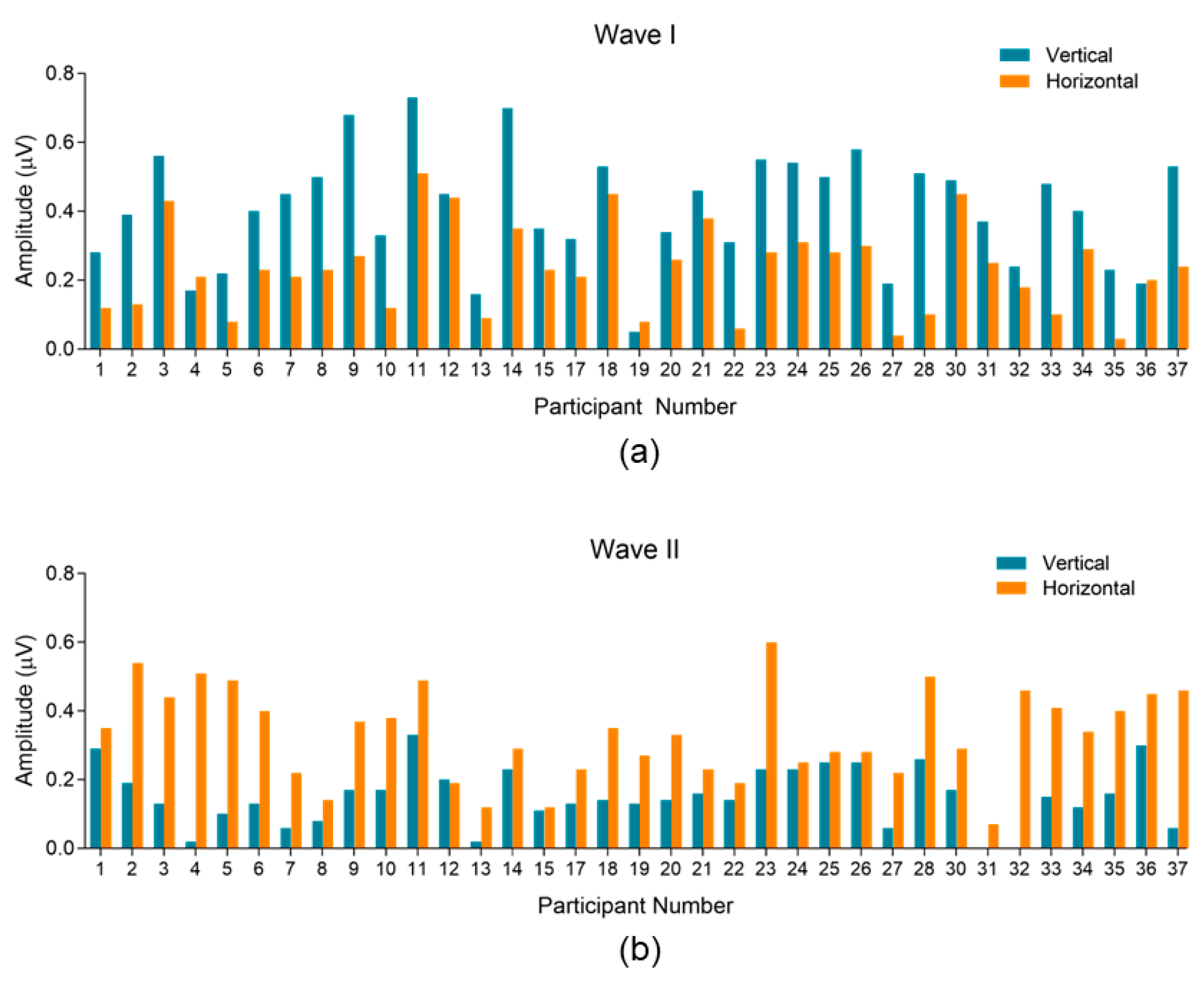
3.2.1. Variability and Trends within Subjects and between Montages
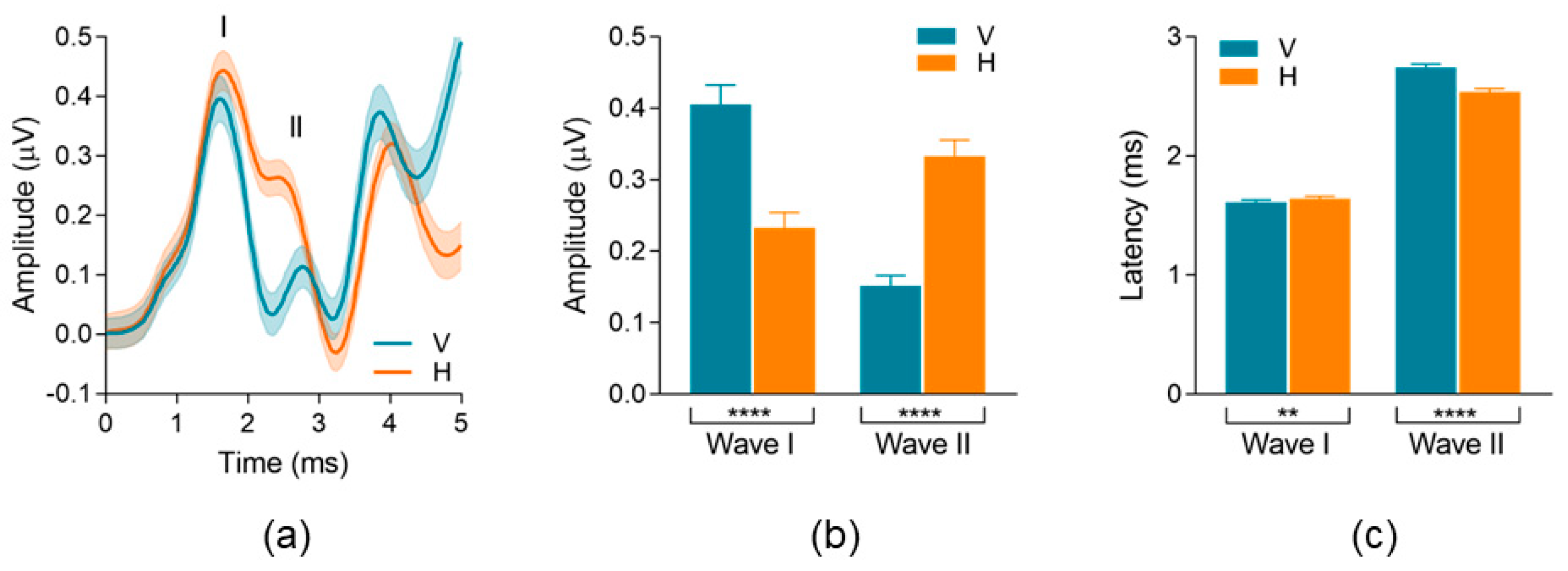
3.2.2. Waveform Peak-to-Trough Amplitude and Peak Latency
3.3. Residual Noise Analysis
4. Discussion
4.1. Standard Electrocochleography (ECochG) Measures
4.2. Montage Differences in Early AEPs: Waves I and II Peak-to-Trough Amplitudes
4.3. Variability Related to Electrode Sites and Types
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [PubMed]
- Sergeyenko, Y.; Lall, K.; Liberman, M.C.; Kujawa, S.G. Age-Related Cochlear Synaptopathy: An Early-Onset Contributor to Auditory Functional Decline. J. Neurosci. 2013, 33, 13686–13694. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear. Res. 2015, 330, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.C.; Bao, J. Optimizing non-invasive functional markers for cochlear deafferentation based on electrocochleography and auditory brainstem responses. J. Acoust. Soc. Am. 2022, 151, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G. Effects of noise exposure on auditory brainstem response and speech-in-noise tasks: A review of the literature. Int. J. Audiol. 2019, 58, S3–S32. [Google Scholar] [CrossRef] [PubMed]
- DiNino, M.; Holt, L.L.; Shinn-Cunningham, B.G. Cutting Through the Noise: Noise-Induced Cochlear Synaptopathy and Individual Differences in Speech Understanding Among Listeners with Normal Audiograms. Ear Hear. 2022, 43, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R.; Jannetta, P.J. Compound action potentials recorded intracranially from the auditory nerve in man. Exp. Neurol. 1981, 74, 862–874. [Google Scholar] [CrossRef]
- Furman, A.C.; Kujawa, S.G.; Liberman, M.C. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol. 2013, 110, 577–586. [Google Scholar] [CrossRef]
- Lobarinas, E.; Spankovich, C.; Le Prell, C.G. Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear. Res. 2017, 349, 155–163. [Google Scholar] [CrossRef]
- Liberman, M.C.; Epstein, M.J.; Cleveland, S.S.; Wang, H.; Maison, S.F. Toward a Differential Diagnosis of Hidden Hearing Loss in Humans. PLoS ONE 2016, 11, e0162726. [Google Scholar] [CrossRef]
- Beattie, R.C. Interaction of click polarity, stimulus level, and repetition rate on the auditory brainstem response. Scand. Audiol. 1988, 17, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Lauter, J.L.; Loomis, R.L. Individual differences in auditory electric responses: Comparisons of between-subject and within-subject variability. II. Amplitude of brainstem Vertex-positive peaks. Scand. Audiol. 1988, 17, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Stockard, J.E.; Stockard, J.J.; Westmoreland, B.F.; Corfits, J.L. Brainstem Auditory-Evoked Responses: Normal Variation as a Function of Stimulus and Subject Characteristics. Arch. Neurol. 1979, 36, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Plack, C.J.; Léger, A.; Prendergast, G.; Kluk, K.; Guest, H.; Munro, K.J. Toward a Diagnostic Test for Hidden Hearing Loss. Trends Hear. 2016, 20, 2331216516657466. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, I.; Ishiyama, Y.; Yoshimoto, T.; Nemoto, S. Brain-Stem Auditory-Evoked Potentials Recorded Directly from Human Brain-Stem and Thalamus. Brain 1981, 104, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R.; Jho, H.D.; Yokota, M.; Jannetta, P.J. Contribution from crossed and uncrossed brainstem structures to the brainstem auditory evoked potentials: A study in humans. Laryngoscope 1995, 105, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, J.A.; Krishnan, G. Cochlear Potentials in Clinical Audiology. Audiol. Neurotol. 1997, 2, 241–256. [Google Scholar] [CrossRef]
- Simpson, M.J.; Jennings, S.G.; Margolis, R.H. Techniques for Obtaining High-quality Recordings in Electrocochleography. Front. Syst. Neurosci. 2020, 14, 18. [Google Scholar] [CrossRef]
- Prendergast, G.; Tu, W.; Guest, H.; Millman, R.E.; Kluk, K.; Couth, S.; Munro, K.J.; Plack, C.J. Supra-threshold auditory brainstem response amplitudes in humans: Test-retest reliability, electrode montage and noise exposure. Hear. Res. 2018, 364, 38–47. [Google Scholar] [CrossRef]
- Singh, C.B.; Mason, S.M. Simultaneous recording of extra-tympanic electrocochleography and brainstem evoked responses in clinical practice. J. Laryngol. Amp Otol. 1981, 95, 279–290. [Google Scholar] [CrossRef]
- Scherg, M.; von Cramon, D. A new interpretation of the generators of BAEP waves I-V: Results of a spatio-temporal dipole model. Electroencephalogr. Clin. Neurophysiol. 1985, 62, 290–299. [Google Scholar] [CrossRef]
- Ruth, R.A.; Hildebrand, D.L.; Cantrell, R.W. A Study of Methods Used to Enhance Wave I in the Auditory Brain Stem Response. Otolaryngol. Head. Neck Surg. 1982, 90, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Keesling, D.A.; Parker, J.P.; Sanchez, J.T. A Comparison of Commercially Available Auditory Brainstem Response Stimuli at a Neurodiagnostic Intensity Level. Audiol. Res. 2017, 7, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, J.A.; Durrant, J.D. Electrocochleography in the evaluation of patients with Ménière’s disease/endolymphatic hydrops. J. Am. Acad. Audiol. 2006, 17, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.J.; Mepani, A.M.; Wu, P.; Hancock, K.E.; de Gruttola, V.; Liberman, M.C.; Maison, S.F. Electrophysiological markers of cochlear function correlate with hearing-in-noise performance among audiometrically normal subjects. J. Neurophysiol. 2020, 124, 418–431. [Google Scholar] [CrossRef]
- Ananthanarayan, A.K.; Durrant, J.D. On the Origin of Wave II of the Auditory Brain Stem Evoked Response. Ear Hear. 1991, 12, 174–179. [Google Scholar] [CrossRef]
- Møller, A.R.; Jannetta, P.; Bennett, M.; Møller, M.B. Intracranially recorded responses from the human auditory nerve: New insights into the origin of brain stem evoked potentials (BSEPs). Electroencephalogr. Clin. Neurophysiol. 1981, 52, 18–27. [Google Scholar] [CrossRef]
- Bramhall, N.F. Use of the auditory brainstem response for assessment of cochlear synaptopathy in humans. J. Acoust. Soc. Am. 2021, 150, 4440–4451. [Google Scholar] [CrossRef]
- Lefler, S.M.; Kaf, W.A.; Ferraro, J.A. Comparing Simultaneous Electrocochleography and Auditory Brainstem Response Measurements Using Three Different Extratympanic Electrodes. J. Am. Acad. Audiol. 2021, 32, 339–346. [Google Scholar] [CrossRef]
- Ferraro, J.A. Electrocochleography: A Review of Recording Approaches, Clinical Applications, and New Findings in Adults and Children. J. Am. Acad. Audiol. 2010, 21, 145–152. [Google Scholar] [CrossRef]
| Montage | Median | Mean (SD) | Min–Max | |
|---|---|---|---|---|
| SPBASE p = 0.25 | Vertical | 0.10 | 0.11 (0.06) | 0.01–0.27 |
| Horizontal | 0.13 | 0.12 (0.06) | 0.02–0.27 | |
| APBASE p = 0.09 | Vertical | 0.42 | 0.41 (0.14) | 0.17–0.68 |
| Horizontal | 0.50 | 0.45 (0.13) | 0.21–0.67 | |
| SP/AP Ratio p = 0.35 | Vertical | 0.24 | 0.25 (0.10) | 0.06–0.55 |
| Horizontal | 0.30 | 0.28 (0.13) | 0.04–0.69 |
| Wave | Montage | Median | Mean (SD) | Min–Max | |
|---|---|---|---|---|---|
| Peak-to-Trough Amplitude (μV) | AP/Wave I p < 0.0001 | Vertical | 0.40 | 0.40 (0.16) | 0.05–0.73 |
| Horizontal | 0.23 | 0.23 (0.13) | 0.03–0.51 | ||
| Wave II p < 0.0001 | Vertical | 0.14 | 0.15 (0.08) | 0.00–0.33 | |
| Horizontal | 0.34 | 0.33 (0.13) | 0.07–0.60 | ||
| Peak Latency (ms) | AP/Wave I p = 0.002 | Vertical | 1.63 | 1.61 (0.10) | 1.35–1.90 |
| Horizontal | 1.65 | 1.64 (0.13) | 1.35–1.98 | ||
| Wave II p < 0.0001 | Vertical | 2.77 | 2.74 (0.18) | 2.25–3.03 | |
| Horizontal | 2.58 | 2.54 (0.16) | 2.23–2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McFarlane, K.A.; Sanchez, J.T. Exploring Electrode Placements to Optimize the Identification and Measurement of Early Auditory Evoked Potentials. Audiol. Res. 2023, 13, 978-988. https://doi.org/10.3390/audiolres13060085
McFarlane KA, Sanchez JT. Exploring Electrode Placements to Optimize the Identification and Measurement of Early Auditory Evoked Potentials. Audiology Research. 2023; 13(6):978-988. https://doi.org/10.3390/audiolres13060085
Chicago/Turabian StyleMcFarlane, Kailyn A., and Jason Tait Sanchez. 2023. "Exploring Electrode Placements to Optimize the Identification and Measurement of Early Auditory Evoked Potentials" Audiology Research 13, no. 6: 978-988. https://doi.org/10.3390/audiolres13060085
APA StyleMcFarlane, K. A., & Sanchez, J. T. (2023). Exploring Electrode Placements to Optimize the Identification and Measurement of Early Auditory Evoked Potentials. Audiology Research, 13(6), 978-988. https://doi.org/10.3390/audiolres13060085






