Acute Otitis Media and Facial Paralysis in Children: A Systemic Review and Proposal of an Operative Algorithm
Abstract
1. Introduction
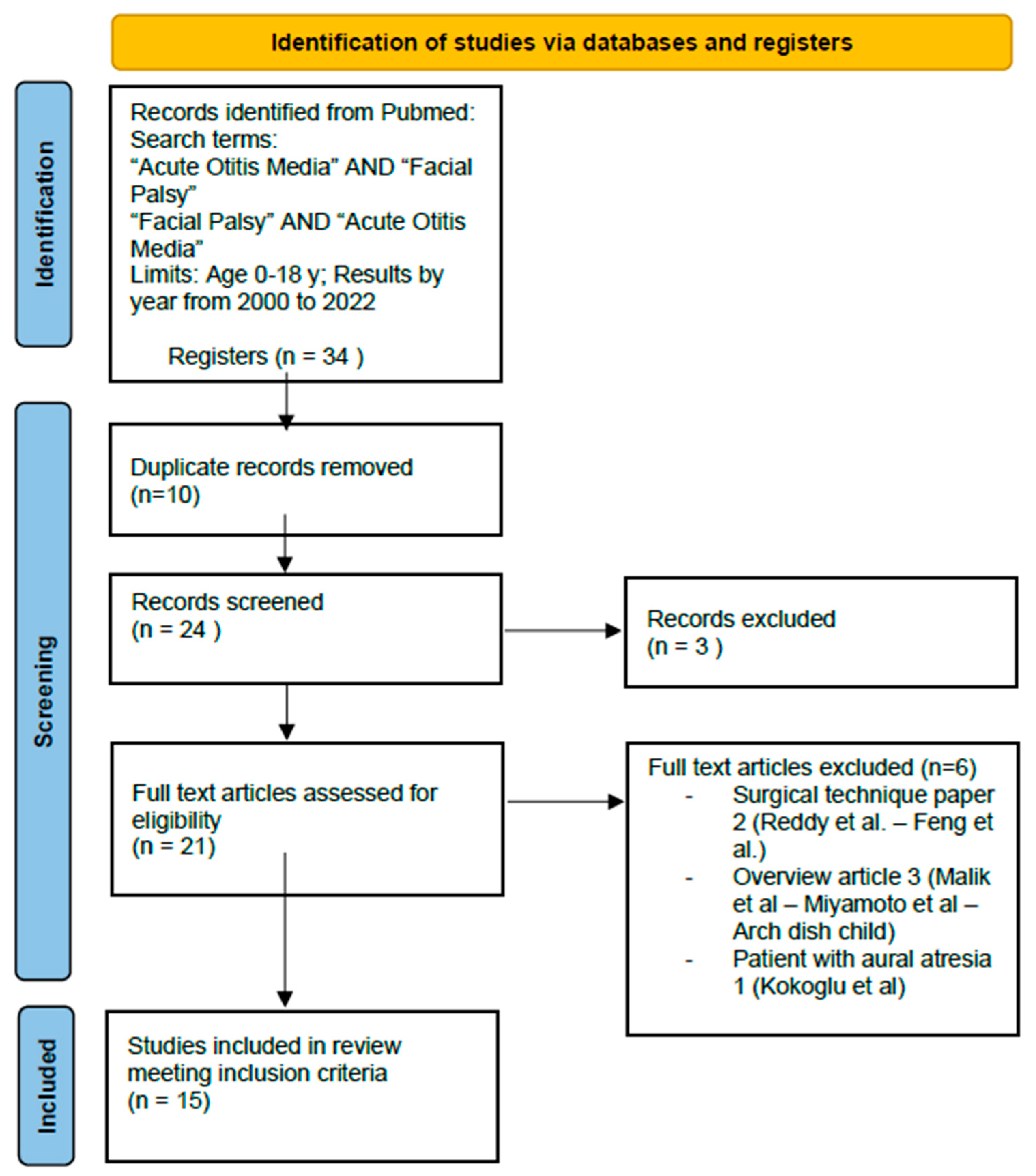
2. Material and Methods
- −
- Written in English;
- −
- Studies published between 2000 and 2022;
- −
- Studies about pediatric patients (age: 0–18 years old);
- −
- Studies using House–Brackmann score.
3. Results
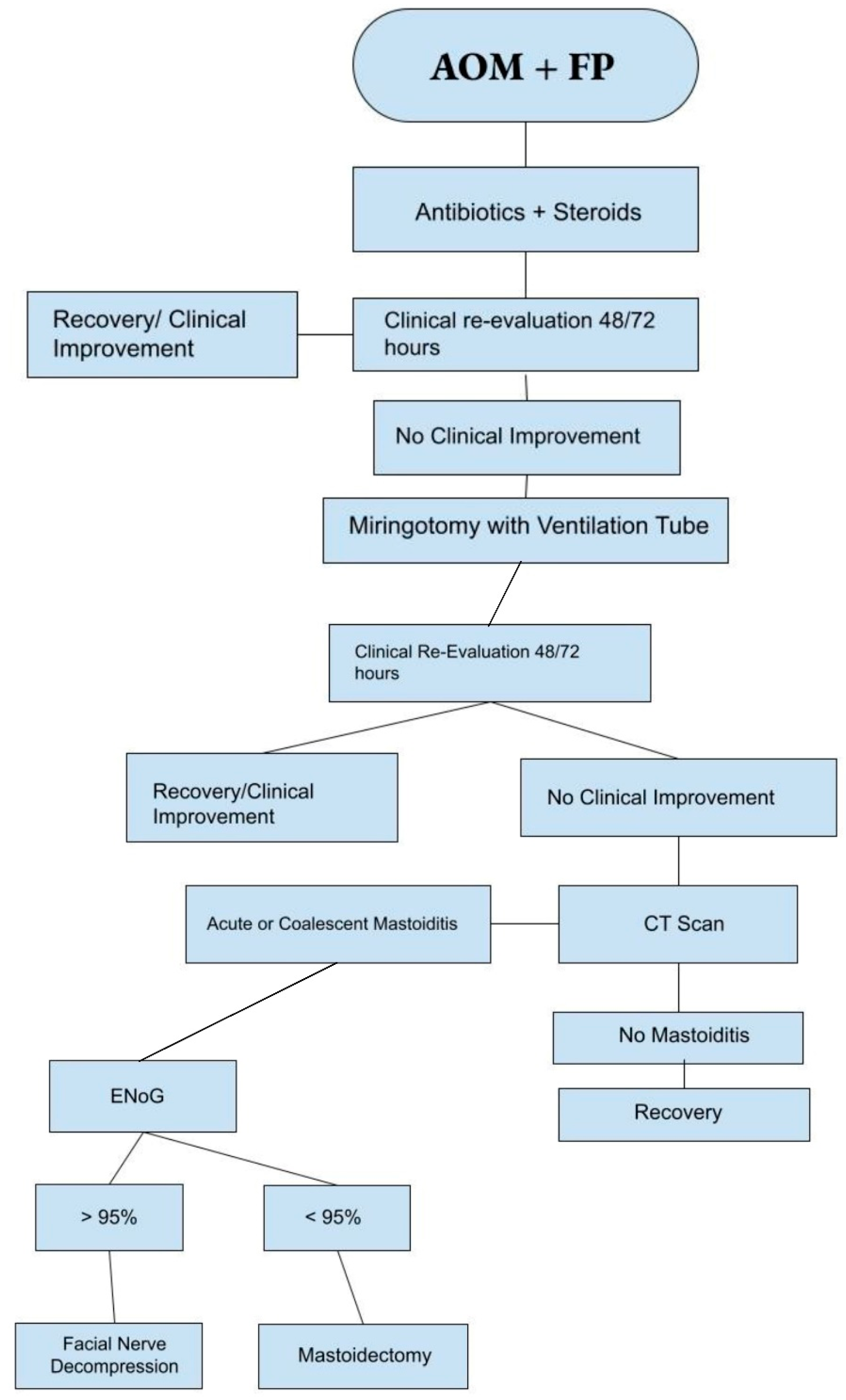
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gaio, E.; Marioni, G.; de Filippis, C.; Tregnaghi, A.; Caltran, S.; Staffieri, A. Facial nerve paralysis secondary to acute otitis media in infants and children. Child Health 2004, 40, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Venekamp, R.P.; Schilder, A.G.M.; Heuvel, M.v.D.; Hay, A.D. Acute otitis media in children. BMJ Clin. Updates 2020, 371, m4238. [Google Scholar] [CrossRef]
- Chonmaitree, T.; Alvarez-Fernandez, P.; Jennings, K.; Trujillo, R.; Marom, T.; Loeffelholz, M.J.; Miller, A.L.; McCormick, D.P.; Patel, J.A.; Pyles, R.B. Symptomatic and asymptomatic respiratory viral infections in the first year of life: Association with acute otitis media development. Clin. Infect. Dis. 2015, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vogelnik, K.; Matos, A. Facial nerve palsy secondary to Epstein–Barr virus infection of the middle ear in pediatric population may be more common than we think. Wien. Klin. Wochenschr. 2017, 129, 844–847. [Google Scholar] [CrossRef]
- Chonmaitree, T.; Ruohola, A.; Hendley, J.O. Presence of viral nucleic acids in the middle ear: Acute otitis media pathogen or bystander? Pediatr. Infect. Dis. J. 2012, 31, 325–330. [Google Scholar] [CrossRef]
- Sakulchit, T.; Goldman, R.D. Antibiotic therapy for children with acute otitis media. Can. Fam. Physician Clin. Updates 2017, 63, 685–687. [Google Scholar]
- Simon, F.; Haggard, M.; Rosenfeld, R.; Jia, H.; Peer, S.; Calmels, M.-N.; Couloigner, V.; Teissier, N. International consensus (ICON) on management of otitis media with effusion in children. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, S33–S39. [Google Scholar] [CrossRef]
- Yonamine, F.K.; Tuma, J.; da Silva, R.F.N.; Soares, M.C.M.; Testa, J.R.G. Facial paralysis associated with acute otitis media. Braz. J. Otorhinolaryngol. 2009, 75, 228–230. [Google Scholar] [CrossRef]
- de Zinis, L.O.R.; Gamba, P.; Balzanelli, C. Acute Otitis Media and Facial Nerve Paralysis in Adults. Otol. Neurotol. 2003, 24, 113–117. [Google Scholar] [CrossRef]
- Castellazzi, M.L.; Torretta, S.; Di Pietro, G.M.; Ciabatta, A.; Capaccio, P.; Caschera, L.; Marchisio, P. Acute otitis media-related facial nerve palsy in a child: A case report and a literary review. Ital. J. Pediatr. 2023, 49, 8. [Google Scholar] [CrossRef]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Harmes, K.M.; Blackwood, R.A.; Burrows, H.L.; Cooke, J.; Van Harrison, R.; Passamani, P.P. Otitis Media: Diagnosis and Treatment. Am. Fam. Physician 2013, 88, 435–440. [Google Scholar] [PubMed]
- Yang, S.H.; Park, H.; Yoo, D.S.; Joo, W.; Rhoton, A. Microsurgical Anatomy of the Facial nerve. Clin. Anotomy 2020, 34, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.R.; Mistry, R.K.; Mazzoni, T. Facial Nerve Palsy; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- White, N.; McCans, K. Facial paralysis secondary to acute otitis media. Pediatr. Emerg. Care 2000, 16, 343–345. [Google Scholar] [CrossRef]
- Schilder, A.G.M.; Chonmaitree, T.; Cripps, A.W.; Rosenfeld, R.M.; Casselbrant, M.L.; Haggard, M.P.; Venekamp, R.P. Otitis media. Nat. Rev. Dis. Primers 2016, 2, 16063. [Google Scholar] [CrossRef]
- Hostetler, M.A.; Suara, R.O.; Denison, M.R. Unilateral facial paralysis occurring in an infant with enteroviral otitis media and aseptic meningitis. J. Emerg. Med. 2002, 22, 267–271. [Google Scholar] [CrossRef]
- Lieberthal, A.S.; Carroll, A.E.; Chonmaitree, T.; Ganiats, T.G.; Hoberman, A.; Jackson, M.A.; Joffe, M.D.; Miller, D.T.; Rosenfeld, R.M.; Sevilla, X.D.; et al. The Diagnosis and Management of Acute Otitis Media. Pediatrics 2013, 131, e964–e999. [Google Scholar] [CrossRef]
- Bradley, M.; Bacharouch, A.; Hart-Johnson, T.; Burrows, H.L.; Blackwood, R.A. Adopting otitis media practice guidelines increases adherence within a large primary care network. J. Paediatr. Child Health 2021, 57, 1054–1059. [Google Scholar] [CrossRef]
- Marchisio, P.; Galli, L.; Bortone, B.; Ciarcià, M.; Motisi, M.A.; Novelli, A.; Pinto, L.; Bottero, S.; Pignataro, L.; Piacentini, G.; et al. Updated Guidelines for the Management of Acute Otitis Media in Children by the Italian Society of Pediatrics. Pediatr. Infect. Dis. 2019, 38, S10–S21. [Google Scholar] [CrossRef]
- Popovtzer, A.; Raveh, E.; Bahar, G.; Oestreicher-Kedem, Y.; Feinmesser, R.; Nageris, B.I. Facial palsy associated with acute otitis media. Otolaryngol.—Head Neck Surg. 2005, 132, 327–329. [Google Scholar] [CrossRef]
- Shokri, T.; Saadi, R.; Schaefer, E.W.; Lighthall, J.G. Trends in the Treatment of Bell’s Palsy. Facial Plast. Surg. 2020, 36, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Ranakusuma, R.W.; Pitoyo, Y.; Safitri, E.D.; Thorning, S.; Beller, E.M.; Sastroasmoro, S.; Del Mar, C.B. Systemic corticosteroids for acute otitis media in children. Cochrane Database Syst. Rev. 2018, 2018, CD012289. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Cubitt, J.J. Paediatric facial paralysis: An overview and insights into management. J. Paediatr. Child Health 2021, 57, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Wohrer, D.; Moulding, T.; Titomanlio, L.; Lenglart, L. Acute Facial Nerve Palsy in Children: Gold Standard Management. Children 2022, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Psillas, G.; Antoniades, E.; Ieridou, F.; Constantinidis, J. Facial nerve palsy in children: A retrospective study of 124 cases. J. Paediatr. Child Health 2018, 55, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, M.; Tjellstrom, A. Acute Otitis Media and Facial Palsy in Children. Acta Prediatr. Scand. 1990, 79, 118–120. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, C.; DIplomatico, M.; Tipo, V. Facial palsy in a baby with acute otitis media. Arch. Dis. Child. Educ. Pract. Ed. 2018, 103, 155–157. [Google Scholar] [CrossRef]
- Riordan, M. Investigation and treatment of facial paralysis. Arch. Dis. Child 2001, 84, 286–288. [Google Scholar] [CrossRef]
- Hydén, D.; Åkerlind, B.; Peebo, M. Inner ear and facial nerve complications of acute otitis media with focus on bacteriology and virology. Acta Oto-Laryngol. 2006, 126, 460–466. [Google Scholar] [CrossRef]
- Evans, A.K.; Licameli, G.; Brietzke, S.; Whittemore, K.; Kenna, M. Pediatric facial nerve paralysis: Patients, management and outcomes. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 1521–1528. [Google Scholar] [CrossRef]

| Grade | Description | Clinical Features |
|---|---|---|
| I | Normal | No paresis |
| II | Mild paresis | No abnormalities at rest |
| III | Moderate paresis | No deformity at rest. Facial synkinesis. With maximum effort, the patient can completely close their eyelids. |
| IV | Moderate–severe paresis | Obvious asymmetry. Facial synkinesis. Even with maximum effort, the patient cannot completely close their eyelids. |
| V | Severe paresis | Obvious asymmetry at rest (ptosis of labial commissure, disappearance of the nasolabial fold). Incomplete eyelid closure. Asymmetry in mouth motion. |
| VI | Complete paralysis | Atony at rest. No facial function. |
| Surgical Procedures | Patients (%) |
|---|---|
| Miringotomy +/− Ventilation tube | 55/120 (45.83%) |
| Mastoidectomy | 7/120 (5.83%) |
| Mastoidectomy with facial nerve decompression | 8/120 (6.66%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fichera, P.; Bruschini, L.; Berrettini, S.; Capobianco, S.; Fiacchini, G. Acute Otitis Media and Facial Paralysis in Children: A Systemic Review and Proposal of an Operative Algorithm. Audiol. Res. 2023, 13, 889-897. https://doi.org/10.3390/audiolres13060077
Fichera P, Bruschini L, Berrettini S, Capobianco S, Fiacchini G. Acute Otitis Media and Facial Paralysis in Children: A Systemic Review and Proposal of an Operative Algorithm. Audiology Research. 2023; 13(6):889-897. https://doi.org/10.3390/audiolres13060077
Chicago/Turabian StyleFichera, Piergabriele, Luca Bruschini, Stefano Berrettini, Silvia Capobianco, and Giacomo Fiacchini. 2023. "Acute Otitis Media and Facial Paralysis in Children: A Systemic Review and Proposal of an Operative Algorithm" Audiology Research 13, no. 6: 889-897. https://doi.org/10.3390/audiolres13060077
APA StyleFichera, P., Bruschini, L., Berrettini, S., Capobianco, S., & Fiacchini, G. (2023). Acute Otitis Media and Facial Paralysis in Children: A Systemic Review and Proposal of an Operative Algorithm. Audiology Research, 13(6), 889-897. https://doi.org/10.3390/audiolres13060077








