Bedside Testing in Acute Vestibular Syndrome—Evaluating HINTS Plus and Beyond—A Critical Review
Abstract
:1. Introduction
2. Bedside Examination Tools in AVS
2.1. HINTS/HINTS Plus
2.2. STANDING
2.3. TriAGe+ Score and PCI-Score
2.4. ABCD2 Score
2.5. Gait and Truncal Instability (GTI) Rating
3. Discussion
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerber, K.A.; Meurer, W.J.; West, B.T.; Fendrick, A.M. Dizziness presentations in U.S. emergency departments, 1995–2004. Acad. Emerg. Med. 2008, 15, 744–750. [Google Scholar] [CrossRef]
- Goeldlin, M.; Gaschen, J.; Kammer, C.; Comolli, L.; Bernasconi, C.A.; Spiegel, R.; Bassetti, C.L.; Exadaktylos, A.K.; Lehmann, B.; Mantokoudis, G.; et al. Frequency, aetiology, and impact of vestibular symptoms in the emergency department: A neglected red flag. J. Neurol. 2019, 266, 3076–3086. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Hsieh, Y.H.; Camargo, C.A., Jr.; Pelletier, A.J.; Butchy, G.T.; Edlow, J.A. Spectrum of dizziness visits to US emergency departments: Cross-sectional analysis from a nationally representative sample. Mayo Clin. Proc. 2008, 83, 765–775. [Google Scholar] [CrossRef]
- Ljunggren, M.; Persson, J.; Salzer, J. Dizziness and the Acute Vestibular Syndrome at the Emergency Department: A Population-Based Descriptive Study. Eur. Neurol. 2018, 79, 5–12. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Camargo, C.A., Jr.; Hsieh, Y.H.; Pelletier, A.J.; Edlow, J.A. Disconnect between charted vestibular diagnoses and emergency department management decisions: A cross-sectional analysis from a nationally representative sample. Acad. Emerg. Med. 2009, 16, 970–977. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; McDonald, K.M.; Meltzer, D.O. How much diagnostic safety can we afford, and how should we decide? A health economics perspective. BMJ Qual. Saf. 2013, 22 (Suppl. 2), ii11–ii20. [Google Scholar] [CrossRef] [PubMed]
- Saber Tehrani, A.S.; Coughlan, D.; Hsieh, Y.H.; Mantokoudis, G.; Korley, F.K.; Kerber, K.A.; Frick, K.D.; Newman-Toker, D.E. Rising annual costs of dizziness presentations to U.S. emergency departments. Acad. Emerg. Med. 2013, 20, 689–696. [Google Scholar] [CrossRef]
- Tarnutzer, A.A.; Lee, S.H.; Robinson, K.A.; Wang, Z.; Edlow, J.A.; Newman-Toker, D.E. ED misdiagnosis of cerebrovascular events in the era of modern neuroimaging: A meta-analysis. Neurology 2017, 88, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Kerber, K.A.; Brown, D.L.; Lisabeth, L.D.; Smith, M.A.; Morgenstern, L.B. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: A population-based study. Stroke 2006, 37, 2484–2487. [Google Scholar] [CrossRef] [PubMed]
- ICD-11 (Mortality and Morbidity Statistics). Available online: https://icd.who.int/dev11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1462112221 (accessed on 18 October 2018).
- Tarnutzer, A.A.; Berkowitz, A.L.; Robinson, K.A.; Hsieh, Y.H.; Newman-Toker, D.E. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ 2011, 183, E571–E592. [Google Scholar] [CrossRef]
- Adams, M.E.; Karaca-Mandic, P.; Marmor, S. Use of Neuroimaging for Patients With Dizziness Who Present to Outpatient Clinics vs Emergency Departments in the US. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.P.; Oliveira, J.E.S.L.; Farah, W.; Seisa, M.; Kara Balla, A.; Christensen, A.; Farah, M.; Hasan, B.; Bellolio, F.; Murad, M.H. Diagnostic accuracy of neuroimaging in emergency department patients with acute vertigo or dizziness: A systematic review and meta-analysis for the Guidelines for Reasonable and Appropriate Care in the Emergency Department. Acad. Emerg. Med. 2022, 80, S56. [Google Scholar] [CrossRef]
- Grewal, K.; Austin, P.C.; Kapral, M.K.; Lu, H.; Atzema, C.L. Missed strokes using computed tomography imaging in patients with vertigo: Population-based cohort study. Stroke 2015, 46, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Tarnutzer, A.A.; Gold, D.; Wang, Z.; Robinson, K.A.; Kattah, J.C.; Mantokoudis, G.; Saber Tehrani, A.S.; Zee, D.S.; Edlow, J.A.; Newman-Toker, D.E. Impact of Clinician Training Background and Stroke Location on Bedside Diagnostic Accuracy in the Acute Vestibular Syndrome—A Meta-Analysis. Ann. Neurol. 2023, 94, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Saber Tehrani, A.S.; Kattah, J.C.; Mantokoudis, G.; Pula, J.H.; Nair, D.; Blitz, A.; Ying, S.; Hanley, D.F.; Zee, D.S.; Newman-Toker, D.E. Small strokes causing severe vertigo: Frequency of false-negative MRIs and nonlacunar mechanisms. Neurology 2014, 83, 169–173. [Google Scholar] [CrossRef]
- Saber Tehrani, A.S.; Kattah, J.C.; Kerber, K.A.; Gold, D.R.; Zee, D.S.; Urrutia, V.C.; Newman-Toker, D.E. Diagnosing Stroke in Acute Dizziness and Vertigo: Pitfalls and Pearls. Stroke 2018, 49, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Newman-Toker, D.E.; Edlow, J.A. TiTrATE: A Novel, Evidence-Based Approach to Diagnosing Acute Dizziness and Vertigo. Neurol. Clin. 2015, 33, 577–599, viii. [Google Scholar] [CrossRef]
- Kattah, J.C.; Talkad, A.V.; Wang, D.Z.; Hsieh, Y.H.; Newman-Toker, D.E. HINTS to diagnose stroke in the acute vestibular syndrome: Three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009, 40, 3504–3510. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Kerber, K.A.; Hsieh, Y.H.; Pula, J.H.; Omron, R.; Saber Tehrani, A.S.; Mantokoudis, G.; Hanley, D.F.; Zee, D.S.; Kattah, J.C. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad. Emerg. Med. 2013, 20, 986–996. [Google Scholar] [CrossRef]
- Vanni, S.; Nazerian, P.; Casati, C.; Moroni, F.; Risso, M.; Ottaviani, M.; Pecci, R.; Pepe, G.; Vannucchi, P.; Grifoni, S. Can emergency physicians accurately and reliably assess acute vertigo in the emergency department? Emerg. Med. Australas 2015, 27, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Carmona, S.; Martinez, C.; Zalazar, G.; Moro, M.; Batuecas-Caletrio, A.; Luis, L.; Gordon, C. The Diagnostic Accuracy of Truncal Ataxia and HINTS as Cardinal Signs for Acute Vestibular Syndrome. Front. Neurol. 2016, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Pan, Q.; Liu, J.; Zhu, Y.; Tan, G.; Zhan, Q.; Zhou, J. Central nystagmus plus ABCD(2) identifying stroke in acute dizziness presentations. Acad. Emerg. Med. 2021, 28, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Gerlier, C.; Hoarau, M.; Fels, A.; Vitaux, H.; Mousset, C.; Farhat, W.; Firmin, M.; Pouyet, V.; Paoli, A.; Chatellier, G.; et al. Differentiating central from peripheral causes of acute vertigo in an emergency setting with the HINTS, STANDING, and ABCD2 tests: A diagnostic cohort study. Acad. Emerg. Med. 2021, 28, 1368–1378. [Google Scholar] [CrossRef]
- Kuroda, R.; Nakada, T.; Ojima, T.; Serizawa, M.; Imai, N.; Yagi, N.; Tasaki, A.; Aoki, M.; Oiwa, T.; Ogane, T.; et al. The TriAGe+ Score for Vertigo or Dizziness: A Diagnostic Model for Stroke in the Emergency Department. J. Stroke Cerebrovasc. Dis. 2017, 26, 1144–1153. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Curthoys, I.S. A clinical sign of canal paresis. Arch. Neurol. 1988, 45, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Edlow, J.A.; Carpenter, C.; Akhter, M.; Khoujah, D.; Marcolini, E.; Meurer, W.J.; Morrill, D.; Naples, J.G.; Ohle, R.; Omron, R.; et al. Guidelines for reasonable and appropriate care in the emergency department 3 (GRACE-3): Acute dizziness and vertigo in the emergency department. Acad. Emerg. Med. 2023, 30, 442–486. [Google Scholar] [CrossRef] [PubMed]
- Dmitriew, C.; Regis, A.; Bodunde, O.; Lepage, R.; Turgeon, Z.; McIsaac, S.; Ohle, R. Diagnostic Accuracy of the HINTS Exam in an Emergency Department: A Retrospective Chart Review. Acad. Emerg. Med. 2021, 28, 387–393. [Google Scholar] [CrossRef]
- Ohle, R.; Montpellier, R.A.; Marchadier, V.; Wharton, A.; McIsaac, S.; Anderson, M.; Savage, D. Can Emergency Physicians Accurately Rule Out a Central Cause of Vertigo Using the HINTS Examination? A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2020, 27, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Vanni, S.; Pecci, R.; Edlow, J.A.; Nazerian, P.; Santimone, R.; Pepe, G.; Moretti, M.; Pavellini, A.; Caviglioli, C.; Casula, C.; et al. Differential Diagnosis of Vertigo in the Emergency Department: A Prospective Validation Study of the STANDING Algorithm. Front. Neurol. 2017, 8, 590. [Google Scholar] [CrossRef]
- Gerlier, C.; Fels, A.; Vitaux, H.; Mousset, C.; Perugini, A.; Chatellier, G.; Ganansia, O. Effectiveness and reliability of the four-step STANDING algorithm performed by interns and senior emergency physicians for predicting central causes of vertigo. Acad. Emerg. Med. 2023, 30, 487–500. [Google Scholar] [CrossRef]
- Edlow, J.A.; Kerber, K. Benign paroxysmal positional vertigo: A practical approach for emergency physicians. Acad. Emerg. Med. 2023, 30, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bahu, A.; Occelli, C.; Thamphya, B.; Levraut, J.; Founier, J.P.; Contenti, J.; Vandersteen, C. Retrospective external validation of the TriAGe+ score to diagnose stroke in emergency department patients presenting with vertigo. Emergencias 2023, 35, 231–232. [Google Scholar] [PubMed]
- Chen, R.; Su, R.; Deng, M.; Liu, J.; Hu, Q.; Song, Z. A Posterior Circulation Ischemia Risk Score System to Assist the Diagnosis of Dizziness. J. Stroke Cerebrovasc. Dis. 2018, 27, 506–512. [Google Scholar] [CrossRef]
- Johnston, S.C.; Rothwell, P.M.; Nguyen-Huynh, M.N.; Giles, M.F.; Elkins, J.S.; Bernstein, A.L.; Sidney, S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007, 369, 283–292. [Google Scholar] [CrossRef]
- Navi, B.B.; Kamel, H.; Shah, M.P.; Grossman, A.W.; Wong, C.; Poisson, S.N.; Whetstone, W.D.; Josephson, S.A.; Johnston, S.C.; Kim, A.S. Application of the ABCD2 score to identify cerebrovascular causes of dizziness in the emergency department. Stroke 2012, 43, 1484–1489. [Google Scholar] [CrossRef]
- Shah, V.P.; Oliveira, J.E.S.L.; Farah, W.; Seisa, M.O.; Balla, A.K.; Christensen, A.; Farah, M.; Hasan, B.; Bellolio, F.; Murad, M.H. Diagnostic accuracy of the physical examination in emergency department patients with acute vertigo or dizziness: A systematic review and meta-analysis for GRACE-3. Acad. Emerg. Med. 2023, 30, 552–578. [Google Scholar] [CrossRef]
- Perloff, M.D.; Patel, N.S.; Kase, C.S.; Oza, A.U.; Voetsch, B.; Romero, J.R. Cerebellar stroke presenting with isolated dizziness: Brain MRI in 136 patients. Am. J. Emerg. Med. 2017, 35, 1724–1729. [Google Scholar] [CrossRef]
- Carmona, S.; Martinez, C.; Zalazar, G.; Koohi, N.; Kaski, D. Acute truncal ataxia without nystagmus in patients with acute vertigo. Eur. J. Neurol. 2023, 30, 1785–1790. [Google Scholar] [CrossRef]
- Honda, S.; Inatomi, Y.; Yonehara, T.; Hashimoto, Y.; Hirano, T.; Ando, Y.; Uchino, M. Discrimination of acute ischemic stroke from nonischemic vertigo in patients presenting with only imbalance. J. Stroke Cerebrovasc. Dis. 2014, 23, 888–895. [Google Scholar] [CrossRef]
- Arch, A.E.; Weisman, D.C.; Coca, S.; Nystrom, K.V.; Wira, C.R., 3rd; Schindler, J.L. Missed Ischemic Stroke Diagnosis in the Emergency Department by Emergency Medicine and Neurology Services. Stroke 2016, 47, 668–673. [Google Scholar] [CrossRef]
- Calic, Z.; Cappelen-Smith, C.; Anderson, C.S.; Xuan, W.; Cordato, D.J. Cerebellar Infarction and Factors Associated with Delayed Presentation and Misdiagnosis. Cerebrovasc. Dis. 2016, 42, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Sangha, N.; Albright, K.C.; Peng, H.; Vahidy, F.; Boehme, A.; Chen, Z.; Savitz, S.I. Misdiagnosis of cerebellar infarctions. Can. J. Neurol. Sci. 2014, 41, 568–571. [Google Scholar] [CrossRef]
- Savitz, S.I.; Caplan, L.R.; Edlow, J.A. Pitfalls in the diagnosis of cerebellar infarction. Acad. Emerg. Med. 2007, 14, 63–68. [Google Scholar] [CrossRef]
- Korda, A.; Wimmer, W.; Zamaro, E.; Wagner, F.; Sauter, T.C.; Caversaccio, M.D.; Mantokoudis, G. Videooculography “HINTS” in Acute Vestibular Syndrome: A Prospective Study. Front. Neurol. 2022, 13, 920357. [Google Scholar] [CrossRef] [PubMed]
- Nham, B.; Reid, N.; Bein, K.; Bradshaw, A.P.; McGarvie, L.A.; Argaet, E.C.; Young, A.S.; Watson, S.R.; Halmagyi, G.M.; Black, D.A.; et al. Capturing vertigo in the emergency room: Three tools to double the rate of diagnosis. J. Neurol. 2022, 269, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Edlow, J.A.; Newman-Toker, D.E.; Savitz, S.I. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008, 7, 951–964. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Curthoys, I.S.; Halmagyi, G.M. Diagnosing Stroke in Acute Vertigo: The HINTS Family of Eye Movement Tests and the Future of the “Eye ECG”. Semin Neurol. 2015, 35, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Sohn, S.I.; Cho, Y.W.; Lee, S.R.; Ahn, B.H.; Park, B.R.; Baloh, R.W. Cerebellar infarction presenting isolated vertigo: Frequency and vascular topographical patterns. Neurology 2006, 67, 1178–1183. [Google Scholar] [CrossRef]
- Moon, I.S.; Kim, J.S.; Choi, K.D.; Kim, M.J.; Oh, S.Y.; Lee, H.; Lee, H.S.; Park, S.H. Isolated nodular infarction. Stroke 2009, 40, 487–491. [Google Scholar] [CrossRef]
- Tarnutzer, A.A.; Dieterich, M. Bedside Examination of the Vestibular and Ocular Motor System in Patients with Acute Vertigo or Dizziness. Clin. Transl. Neurosci. 2019, 3, 19. [Google Scholar] [CrossRef]
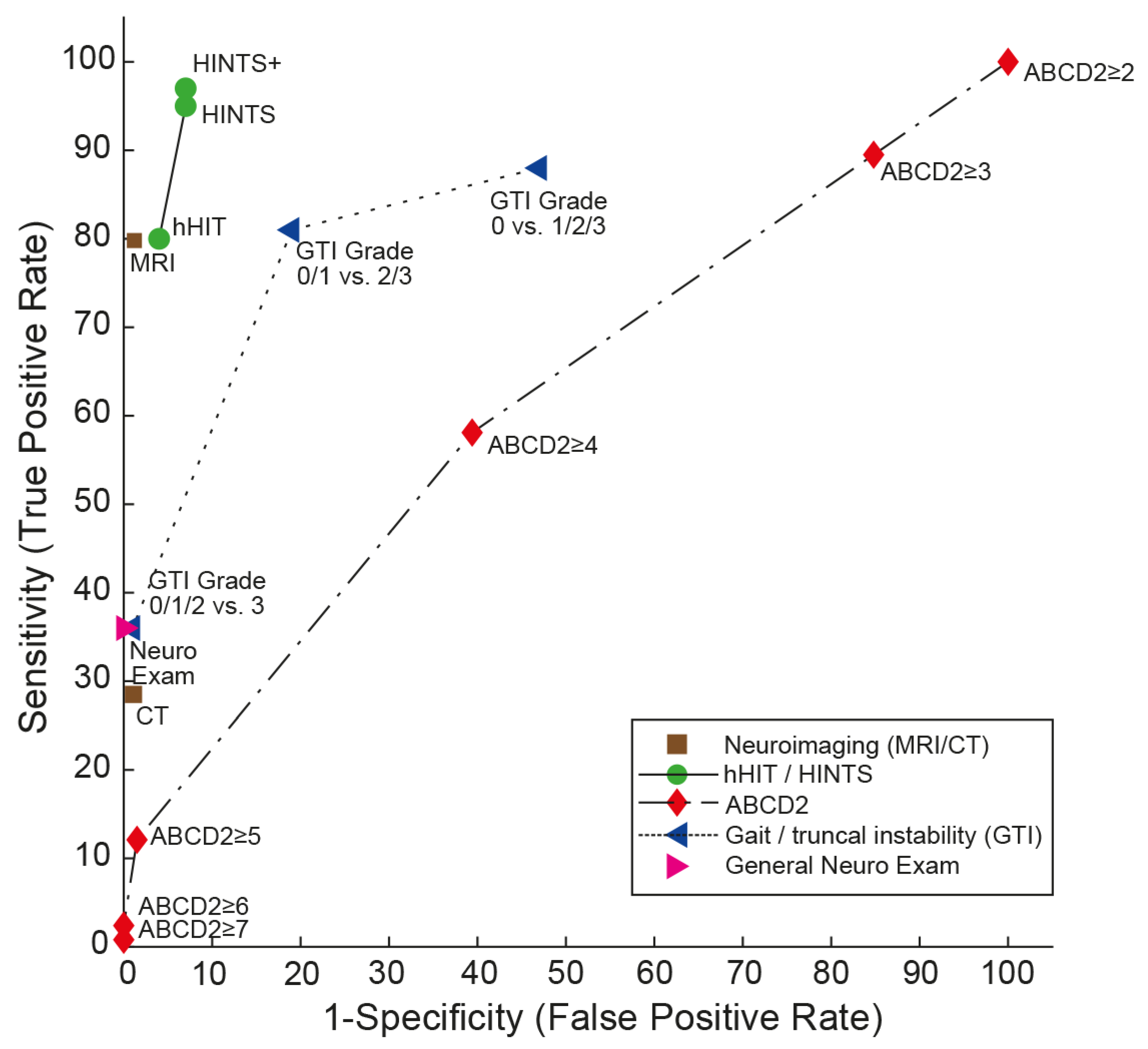
| Score/Algorithm | General Clinical Elements Included | Specific Elements Tested | Evaluated Application | AUC (95% CI) | Sensitivity/Specificity (95% CI) * | Number of Studies Available, at Least One Validation Study Available (Yes/No) | Additional Training Required (Yes/No) | Advantages/Disadvantages |
|---|---|---|---|---|---|---|---|---|
| HINTS [19] | Subtle oculomotor signs | Horizontal head-impulse test, horizontal gaze-evoked nystagmus, test of skew | AVS with nystagmus | 0.995 (0.985–1.000) [20] | 95.3% (92.5–98.1%)/92.6% (88.6–96.5%) [15] | Largest number of studies available (>10 LOE grade 1–3 studies). Validation studies available. | Yes, moderate training is needed (4–6 h [24,26]) for successful application. | High sensitivity and specificity. Only patients with at least one vascular risk factor included in original study [19]. |
| HINTS+ [20] | Subtle oculomotor signs | Horizontal head-impulse test, horizontal gaze-evoked nystagmus, test of skew, finger rub | AVS with nystagmus | NA | 97.2% (94.0–100.0%)/92.4% (86.9–97.9%) [15] | Large number of studies available (6 LOE grade 1–3 studies). Validation studies available. | Yes, moderate training is needed (4–6 h [24,26]) for successful application. | High sensitivity and specificity. Only patients with at least one vascular risk factor included in original study [19]. |
| STANDING [21,27] | Obvious focal neurologic signs and subtle oculomotor signs | Horizontal head-impulse test, horizontal gaze-evoked nystagmus, truncal ataxia, provocation maneuvers (Hallpike Dix, Pagnini–McClure) | Acute vertigo or dizziness | NA | 93.4–100%/71.8%–94.3% [28] | Moderate number of studies available, including 2 LOE grade 1–3 studies from one group). Internal and external validation available. | Yes, moderate training needed (4–6 h [24,26]) for successful application. | More inclusive than HINTS(+), covering positional vertigo (BPPV) also. |
| ABCD2 score [29] | Presenting sx, vascular risk factors, obvious focal neurologic signs | Age, blood pressure, clinical features (unilateral weakness, speech disturbance), duration of symptoms, diabetes | Acute vertigo or dizziness (some studies meeting criteria for AVS) | Range: 0.613 to 0.79 (0.61 (0.53–0.70) [20]; 0.69 (0.63–0.75) [30]; 0.73 (0.68–0.78) [25]; 0.79 (0.73–0.85) [29]) | For a cutoff value of ≥4: 55.7% (43.3–67.5%)/81.8% (76.4–86.2%) [24]; 61.1% (52–70%)/62.3% (51–72%) [20] | Moderate number of studies available, including 2 LOE grade 1–3 studies. Internal and external validation available. | No | Low diagnostic accuracy in acutely dizzy patients. Does not replace other scores such as HINTS or STANDING. |
| TriAGe+ score [25] | Presenting sx, vascular risk factors, obvious focal neurologic signs, subtle oculomotor signs | Triggers, atrial fibrillation, male gender, blood pressure ≥ 140/90 mm Hg, brainstem/cerebellar dysfunction (incl. skew deviation, truncal ataxia), focal weakness or speech impairment, dizziness, no history of vertigo/dizziness, labyrinth/vestibular disease | Acute vertigo or dizziness | 0.82 (0.78–0.86) | For a cutoff value of 10 points: 77.5% (72.8–81.8%)/72.1% (64.1–79.2%), | Single center, retrospective study, with a single retrospective validation study that has serious limitations [31]. | No | Moderate diagnostic accuracy in acutely dizzy patients. Does not replace other scores such as HINTS or STANDING. |
| PCI score [30] | Past history, presenting sx, vascular risk factors, obvious focal neurologic signs | High blood pressure, diabetes mellitus, ischemic stroke, rotating and rocking, difficulty in speech, tinnitus, limb and sensory deficit, gait ataxia, and limb ataxia | Acute vertigo or dizziness | 0.82 (0.77 to 0.87) | For a cutoff value of 0 points: 94.1% (NA)/41.4% (NA) | Single center, retrospective study, no prospective validation studies available. | No | Moderate diagnostic accuracy in acutely dizzy patients (high sensitivity but low specificity). Does not replace other scores such as HINTS or STANDING. |
| GTI rating [22,32,33,34] | Obvious focal neurologic signs | Gait and truncal instability (graded rating) | Acute vertigo, dizziness, or gait imbalance | NA | For a presence of truncal or gait ataxia: 69.7% (43.3–87.9%)/83.7% (52.1–96.0%) [28] | Moderate number of studies available, including 1 LOE 1 study [22]. Internal and external validation available. | No | Lower sensitivity than HINTS(+) or STANDING, but applicable also in patients with isolated truncal instability (without nystagmus) [34]. |
| Test Performed | Property Evaluated | How to Perform This Test | Pointing to a Peripheral Cause | Pointing to a Central Cause | Comments |
|---|---|---|---|---|---|
| Horizontal Head-Impulse test (HIT) | Vestibulo-ocular reflex (VOR) | Fast, low amplitude (10–15°) head rotations to the left/right while the patient is looking at a fixed target in space (e.g., the examiner’s nose) | Delayed to one side, pathological catch-up saccade | Normal HIT. | Note that central lesions involving the VOR (e.g., lesions in the root-entry zone or of the vestibular nuclei) may show a “pseudo-peripheral pattern” |
| Testing for Nystagmus | Eccentric gaze-holding on lateral gaze | Fixation of an object (e.g., the tip of a pen) during lateral (eccentric) gaze (~20 to 30°) for at least 5 s. | Stable eccentric gaze-holding | Deficient eccentric gaze-holding with centripetal drift and centrifugal nystagmus (i.e., left-beating on left-gaze and right-beating on right-gaze). | Spontaneous, predominantly horizontal nystagmus (i.e., primary gaze nystagmus) can be found in both peripheral and central causes and thus allows no differentiation. |
| Alternating cover test (“Test of Skew”) | Vertical alignment of the eyes | Rapid covering then uncovering one eye after the other while the patient is looking at a fixed target in space (e.g., the examiner’s nose). The examiner should focus on only one eye. | No vertical deviation of the eyes | Vertical realignment of the uncovered eye (one eye goes up while the other eye goes down). This is why it does not matter which eye the examiner focuses on. | Note that rarely a vertical skew can also be observed in peripheral-vestibular deficits, but is usually of smaller amplitude and short-lived. |
| New-onset unilateral hearing loss (fourth sign—“plus sign”) | Hearing | Finger rub on each side | Normal hearing | Hearing loss on the side with the abnormal head-impulse test | Hearing may also be compromised in inner ear disorders such as labyrinthitis or complicated otitis media, emphasizing the need for a dedicated examination of the ear. |
| Grade of Gait Inability | Definition |
|---|---|
| 0 | Normal gait |
| 1 | Mild to moderate imbalance but can walk independently [32], or unable to stand on tandem Romberg with the eyes open at least for 3 s [33]. |
| 2 | Severe imbalance with standing and cannot walk without support [32], or unable to stand on tandem Romberg with eyes open for 3 s [33]. |
| 3 | Inability to stand upright unassisted [32,33], or inability to sit upright unassisted [33]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarnutzer, A.A.; Edlow, J.A. Bedside Testing in Acute Vestibular Syndrome—Evaluating HINTS Plus and Beyond—A Critical Review. Audiol. Res. 2023, 13, 670-685. https://doi.org/10.3390/audiolres13050059
Tarnutzer AA, Edlow JA. Bedside Testing in Acute Vestibular Syndrome—Evaluating HINTS Plus and Beyond—A Critical Review. Audiology Research. 2023; 13(5):670-685. https://doi.org/10.3390/audiolres13050059
Chicago/Turabian StyleTarnutzer, Alexander A., and Jonathan A. Edlow. 2023. "Bedside Testing in Acute Vestibular Syndrome—Evaluating HINTS Plus and Beyond—A Critical Review" Audiology Research 13, no. 5: 670-685. https://doi.org/10.3390/audiolres13050059
APA StyleTarnutzer, A. A., & Edlow, J. A. (2023). Bedside Testing in Acute Vestibular Syndrome—Evaluating HINTS Plus and Beyond—A Critical Review. Audiology Research, 13(5), 670-685. https://doi.org/10.3390/audiolres13050059









