Influence of Bone Conduction Hearing Device Implantation on Health-Related Quality of Life for Patients with and without Tinnitus
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Statistical Analysis
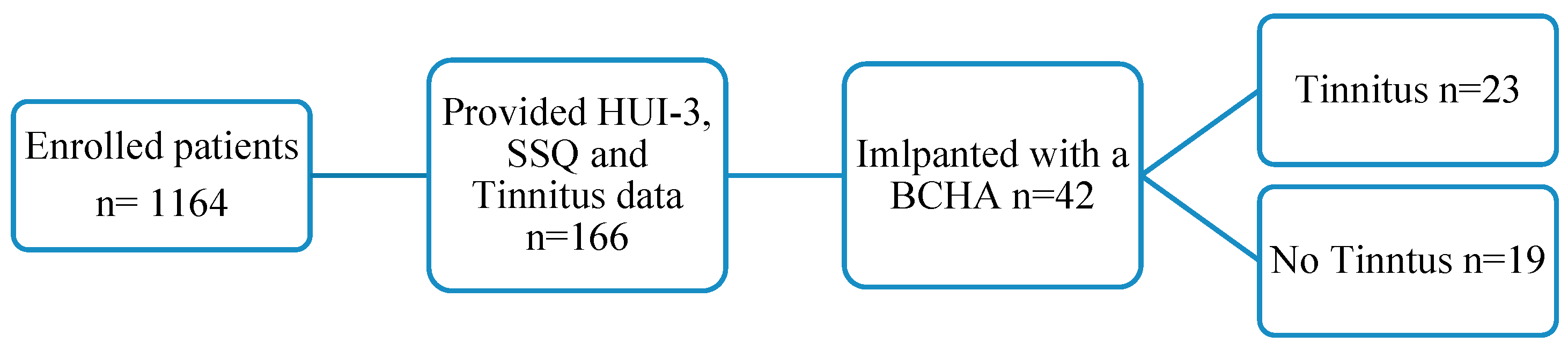
3. Results
3.1. Demographics and Statistics Summary
Study Sample
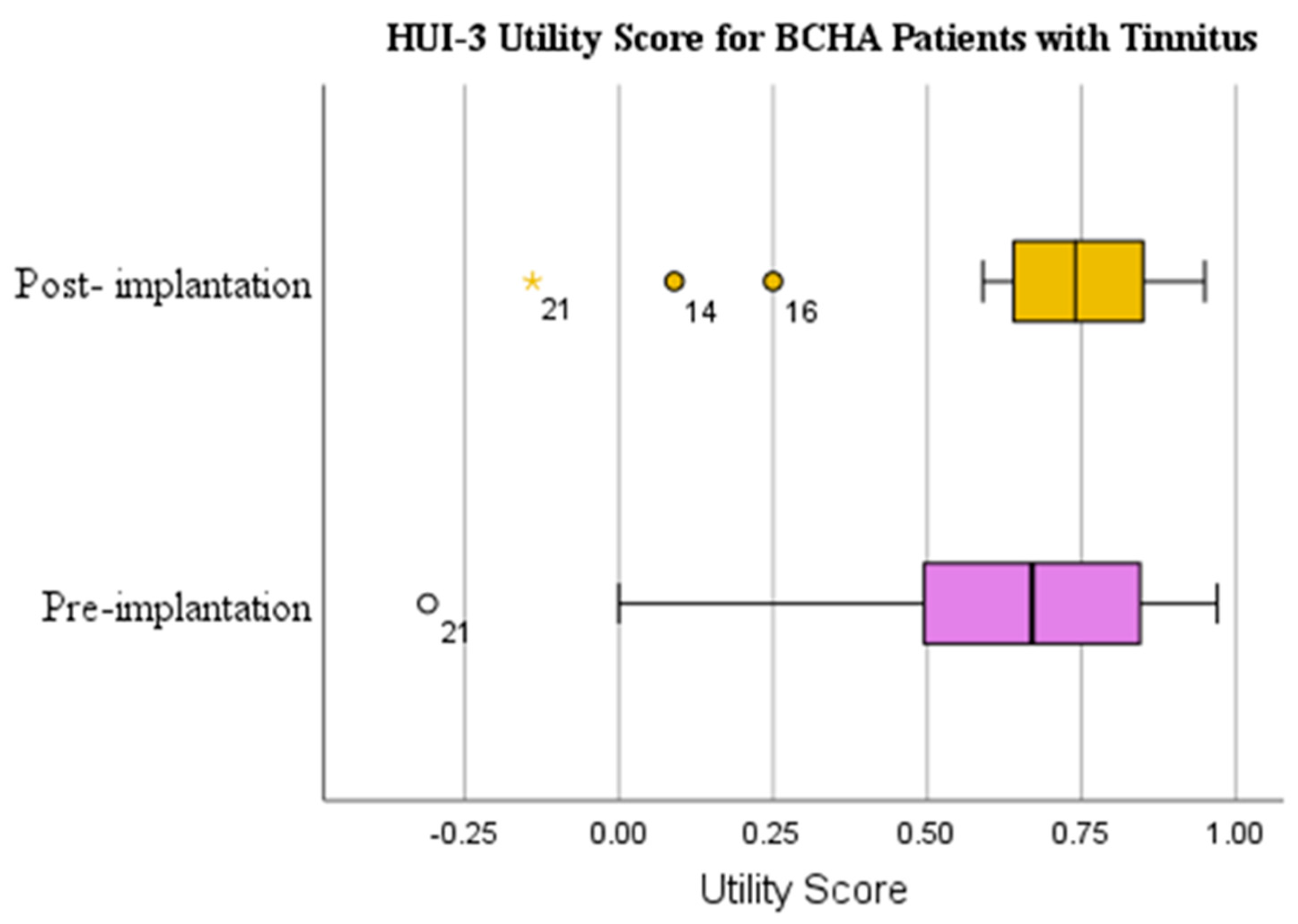
3.2. Bone Conduction Hearing Aid Scores
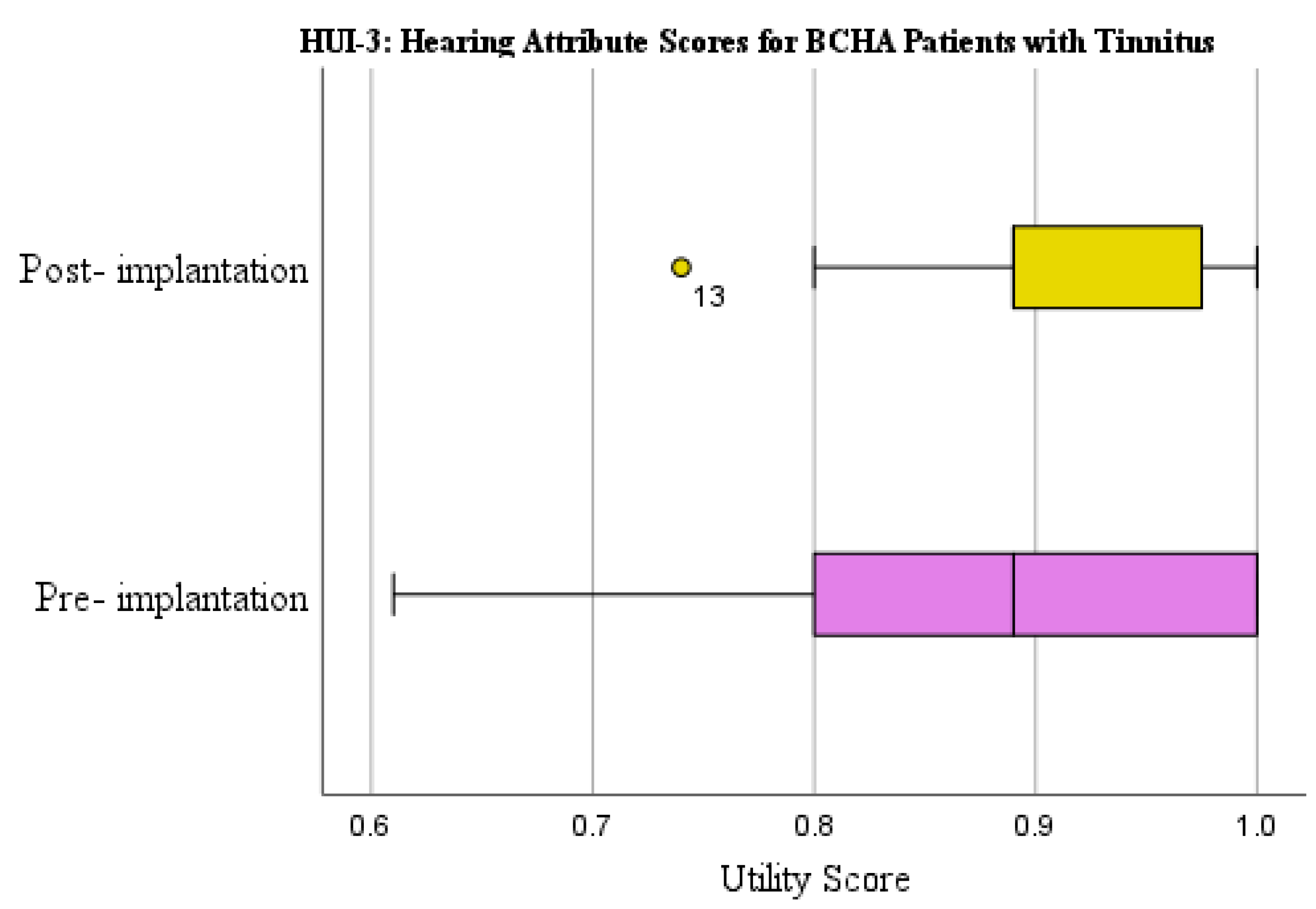
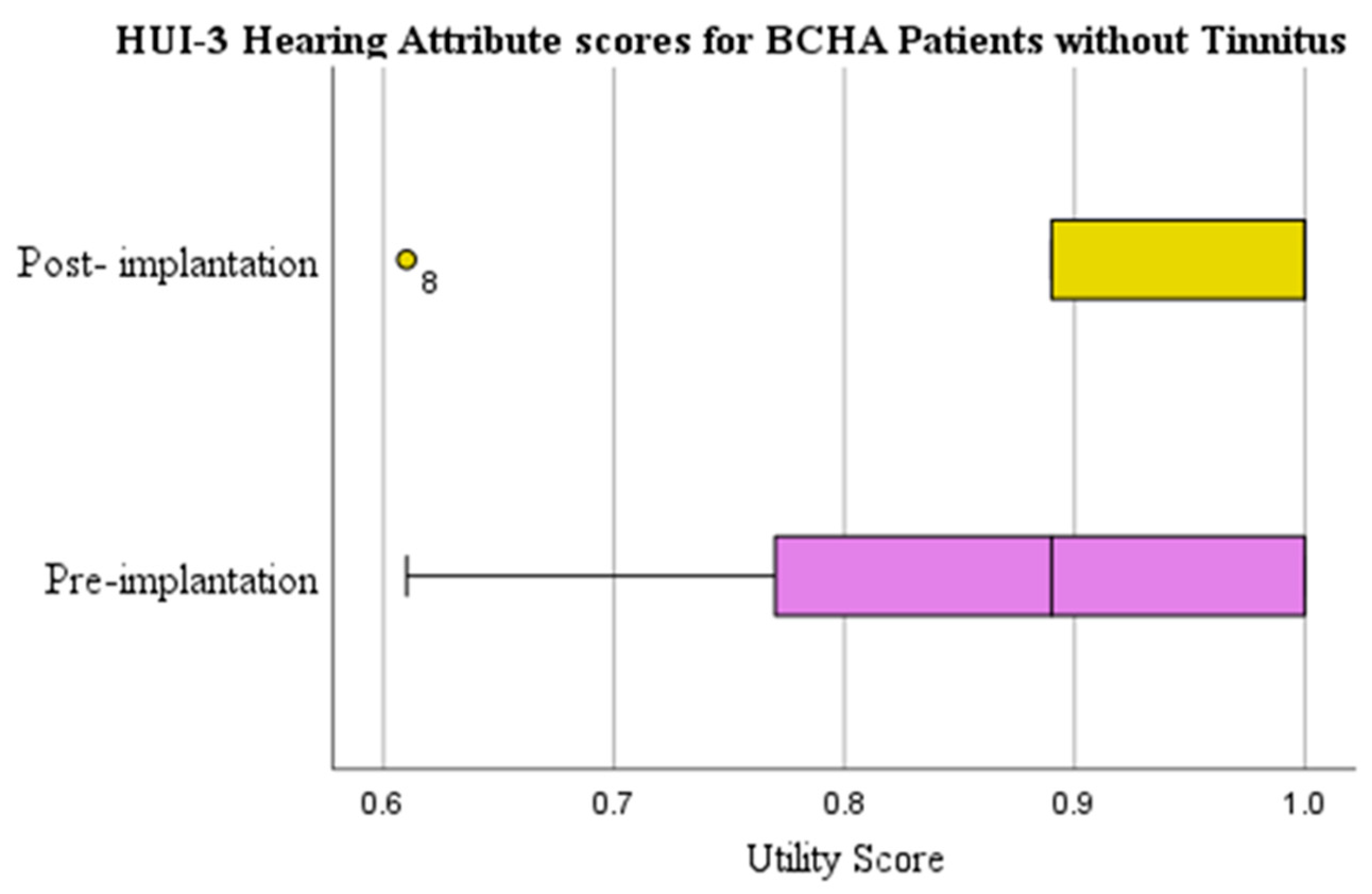
3.2.1. Health Utilities Index Mark 3: Hearing
3.2.2. Speech, Spatial and Qualities of Hearing Scale-49
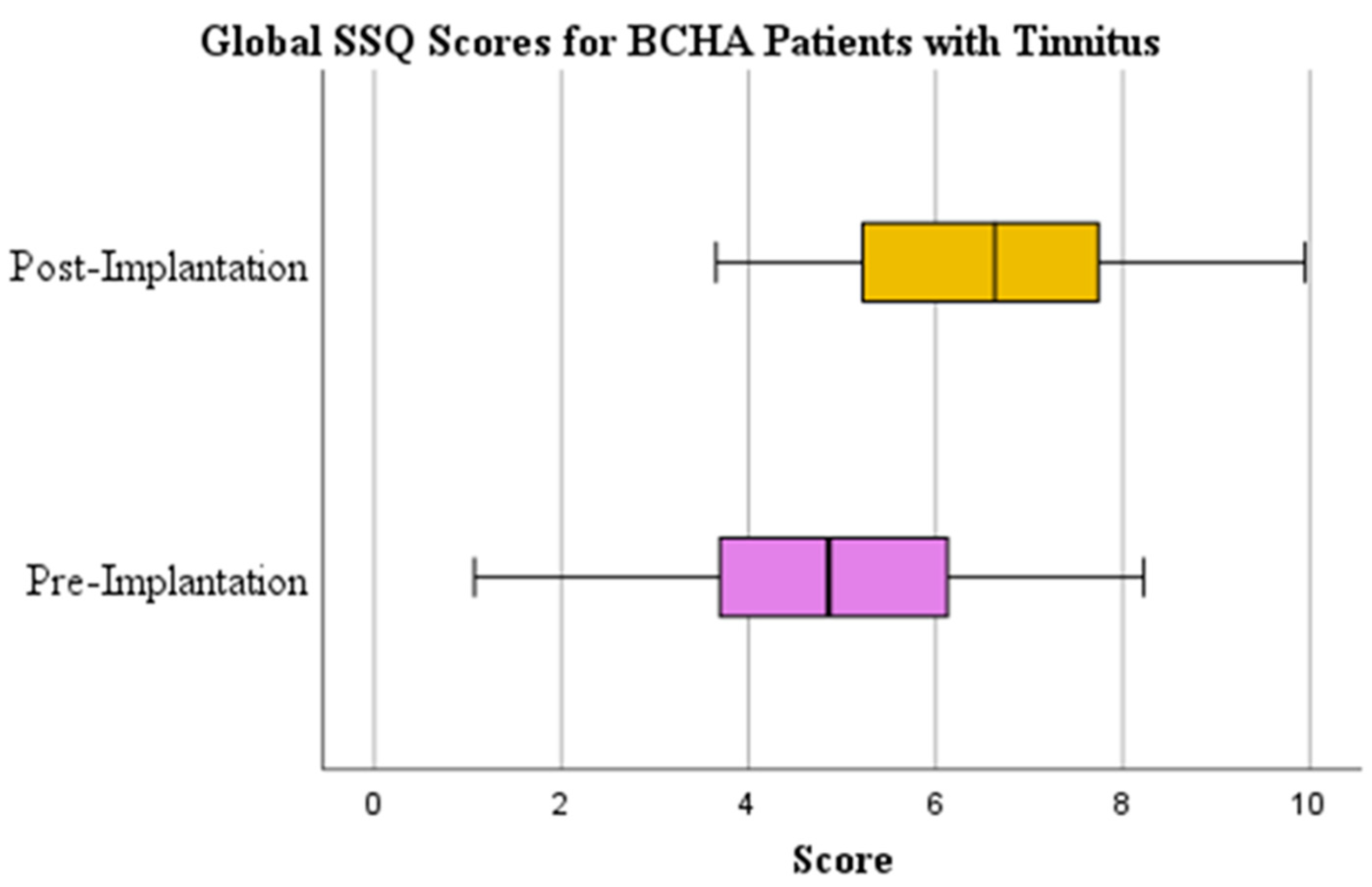
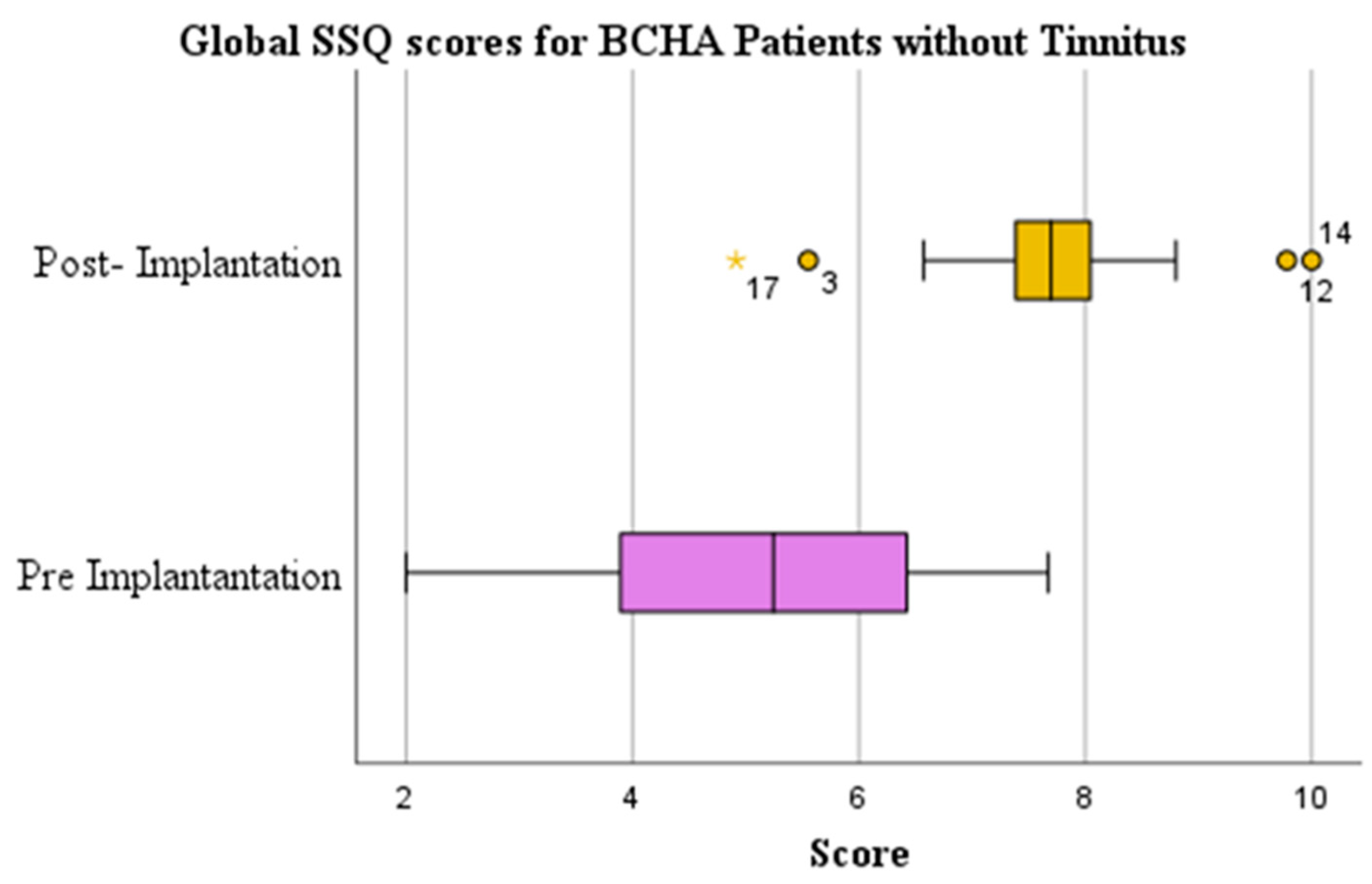
3.3. Tinnitus Perception
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Trotter, M.I.; Donaldson, I. Hearing aids and tinnitus therapy: A 25-year experience. J. Laryngol. Otol. 2008, 122, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.G.; de Medeiros, Í.R.T.; Levy, C.P.D.; da Rosa Oiticica Ramalho, J.; Bento, R.F. Tinnitus in normally hearing patients: Clinical aspects and repercussions. Braz. J. Otorhinolaryngol. 2005, 71, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Oiticica, J.; Bittar, R.S.M. Tinnitus prevalence in the city of São Paulo. Braz. J. Otorhinolaryngol. 2015, 81, 167–176. [Google Scholar] [CrossRef]
- Hébert, S.; Canlon, B.; Hasson, D.; Hanson, L.L.M.; Westerlund, H.; Theorell, T. Tinnitus Severity Is Reduced with Reduction of Depressive Mood—A Prospective Population Study in Sweden. PLoS ONE 2012, 7, e37733. [Google Scholar] [CrossRef]
- Gopinath, B.; McMahon, C.M.; Rochtchina, E.; Karpa, M.J.; Mitchell, P. Incidence, Persistence, and Progression of Tinnitus Symptoms in Older Adults: The Blue Mountains Hearing Study. Ear Hear. 2010, 31, 407–412. [Google Scholar] [CrossRef]
- Baigi, A.; Oden, A.; Almlid-Larsen, V.; Barrenäs, M.-L.; Holgers, K.-M. Tinnitus in the General Population With a Focus on Noise and Stress: A Public Health Study. Ear Hear. 2011, 32, 787–789. [Google Scholar] [CrossRef]
- Axelsson, A.; Ringdahl, A. Tinnitus—A study of its prevalence and characteristics. Br. J. Audiol. 1989, 23, 53–62. [Google Scholar] [CrossRef]
- Shore, S.E.; Roberts, L.E.; Langguth, B. Maladaptive plasticity in tinnitus—Triggers, mechanisms and treatment. Nat. Rev. Neurol. 2016, 12, 150–160. [Google Scholar] [CrossRef]
- Coles, R.; Davis, A.; Smith, P. (Eds.) Tinnitus: Its epidemiology and management. In Presbyacusis and Other Age Related Aspects, Proceedings of the 14th Danavox Symposium; Danavox Jubilee Foundation: Copenhagen, Denmark, 1990. [Google Scholar]
- Krog, N.H.; Engdahl, B.; Tambs, K. The association between tinnitus and mental health in a general population sample: Results from the HUNT Study. J. Psychosom. Res. 2010, 69, 289–298. [Google Scholar] [CrossRef]
- Bhatt, J.M.; Bhattacharyya, N.; Lin, H.W. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 2017, 127, 466–469. [Google Scholar] [CrossRef]
- Sullivan, M.D.; Katon, W.; Dobie, R.; Sakai, C.; Russo, J.; Harrop-Griffiths, J. Disabling tinnitus: Association with affective disorder. Gen. Hosp. Psychiatry 1988, 10, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.A.; Dennis, K.C.; Schechter, M.A. General Review of Tinnitus. J. Speech Lang. Hear. Res. 2005, 48, 1204–1235. [Google Scholar] [CrossRef] [PubMed]
- Bauch, C.D.; Lynn, S.G.; Williams, D.E.; Mellon, M.W.; Weaver, A.L. Tinnitus Impact: Three Different Measurement Tools. J. Am. Acad. Audiol. 2003, 14, 181–187. [Google Scholar] [CrossRef]
- Happich, M.; von Lengerke, T. Valuing the health state ‘tinnitus’: Differences between patients and the general public. Hear. Res. 2005, 207, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Weise, C.; Heinecke, K.; Rief, W. Biofeedback-based behavioral treatment for chronic tinnitus: Results of a randomized controlled trial. J. Consult. Clin. Psychol. 2008, 76, 1046–1057. [Google Scholar] [CrossRef]
- Trochidis, I.; Lugo, A.; Borroni, E.; Cederroth, C.R.; Cima, R.; Kikidis, D.; Langguth, B.; Schlee, W.; Gallus, S. Systematic Review on Healthcare and Societal Costs of Tinnitus. Int. J. Environ. Res. Public Health 2021, 18, 6881. [Google Scholar] [CrossRef]
- Daoud, E.; Caimino, C.; Akeroyd, M.A.; Noreña, A.J.; Baguley, D.M. The Utility of Economic Measures to Quantify the Burden of Tinnitus in Affected Individuals: A Scoping Review. PharmacoEconomics Open 2021, 6, 21–32. [Google Scholar] [CrossRef]
- Tziridis, K.; Friedrich, J.; Brüeggemann, P.; Mazurek, B.; Schulze, H. Estimation of Tinnitus-Related Socioeconomic Costs in Germany. Int. J. Environ. Res. Public Health 2022, 19, 10455. [Google Scholar] [CrossRef]
- Wilson, C.; Lewis, P.; Stephens, D. The short form 36 (SF36) in a specialist tinnitus clinic: La forma corta 36 (SF36) en una clínica especializada en acüfenos. Int. J. Audiol. 2002, 41, 216–220. [Google Scholar] [CrossRef]
- Arts, R.A.; George, E.L.; Stokroos, R.J.; Vermeire, K. Cochlear implants as a treatment of tinnitus in single-sided deafness. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 398–403. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Hearing. 2021. Available online: https://www.who.int/publications/i/item/world-report-on-hearing (accessed on 1 August 2022).
- Stouffer, J.L.; Tyler, R.S. Characterization of Tinnitus by Tinnitus Patients. J. Speech Hear. Disord. 1990, 55, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Tyler, R.S.; Keiner, A.J.; Walker, K.; Deshpande, A.K.; Witt, S.; Killian, M.J.P.; Ji, H.; Patrick, J.; Dillier, N.; van Dijk, P.; et al. A Series of Case Studies of Tinnitus Suppression With Mixed Background Stimuli in a Cochlear Implant. Am. J. Audiol. 2015, 24, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Ellsperman, S.E.; Nairn, E.M.; Stucken, E.Z. Review of Bone Conduction Hearing Devices. Audiol. Res. 2021, 11, 207–219. [Google Scholar] [CrossRef]
- Janssen, R.M.; Hong, P.; Chadha, N.K. Bilateral Bone-Anchored Hearing Aids for Bilateral Permanent Conductive Hearing Loss. Otolaryngol. Neck Surg. 2012, 147, 412–422. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kahinga, A.A.; Moon, I.S. Clinical effect of an active transcutaneous bone-conduction implant on tinnitus in patients with ipsilateral sensorineural hearing loss. Auris Nasus Larynx 2021, 48, 394–399. [Google Scholar] [CrossRef]
- Galvin, K.L.; Mok, M. Everyday Listening Performance of Children Before and After Receiving a Second Cochlear Implant: Results Using the Parent Version of the Speech, Spatial, and Qualities of Hearing Scale. Ear and hearing. Ear Hear. 2016, 37, 93–102. [Google Scholar] [CrossRef]
- Gatehouse, S.; Noble, W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int. J. Audiol. 2004, 43, 85–99. [Google Scholar] [CrossRef]
- Pennini, P.T.M.; de Almeida, K. Speech, Spatial and Qualities of hearing scale na avaliação do benefício em usuários de prótese auditiva. Codas 2021, 33, e20190196. [Google Scholar] [CrossRef]
- Grootendorst, P.; Feeny, D.; Furlong, W.M. Health Utilities Index Mark 3: Evidence of Construct Validity for Stroke and Arthritis in a Population Health Survey. Med. Care 2000, 38, 290–299. [Google Scholar] [CrossRef]
- Horsman, J.; Furlong, W.; Feeny, D.; Torrance, G. The Health Utilities Index (HUI®): Concepts, measurement properties and applications. Health Qual. Life Outcomes 2003, 1, 54. [Google Scholar] [CrossRef]
- Dixon, P.R.; Feeny, D.; Tomlinson, G.; Cushing, S.; Chen, J.M.; Krahn, M.D. Health-Related Quality of Life Changes Associated With Hearing Loss. JAMA Otolaryngol. Neck Surg. 2020, 146, 630–638. [Google Scholar] [CrossRef]
- Stockdale, D.; McFerran, D.; Brazier, P.; Pritchard, C.; Kay, T.; Dowrick, C.; Hoare, D.J. An economic evaluation of the healthcare cost of tinnitus management in the UK. BMC Health Serv. Res. 2017, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Amoodi, H.A.; Mick, P.T.; Shipp, D.B.; Friesen, L.M.; Nedzelski, J.M.; Chen, J.M.; Lin, V.Y.W. The effects of unilateral cochlear implantation on the tinnitus handicap inventory and the influence on quality of life. Laryngoscope 2011, 121, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Mertens, G.; De Bodt, M.; Van de Heyning, P. Cochlear implantation as a long-term treatment for ipsilateral incapacitating tinnitus in subjects with unilateral hearing loss up to 10 years. Hear. Res. 2016, 331, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mauch, H.; Kaur, J.; Irwin, C.; Wyss, J. Design, implementation, and management of an international medical device registry. Trials 2021, 22, 845. [Google Scholar] [CrossRef] [PubMed]
- Health Utilities Inc. Self-Complete Questionnaire Manual. 2018. Available online: http://www.healthutilities.com/ (accessed on 1 August 2022).
- Aarts, S.; Akker, M.v.D.; Winkens, B. The importance of effect sizes. Eur. J. Gen. Pract. 2014, 20, 61–64. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Kirk, R.E. Practical Significance: A Concept Whose Time Has Come. Educ. Psychol. Meas. 1996, 56, 746–759. [Google Scholar] [CrossRef]
- Ratner, B. The correlation coefficient: Its values range between +1/−1, or do they? J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Drummond, M. Introducing economic and quality of life measurements into clinical studies. Ann. Med. 2001, 33, 344–349. [Google Scholar] [CrossRef]
- Sanderson, G.; Ariyaratne, T.V.; Wyss, J.; Looi, V. A global patient outcomes registry: Cochlear paediatric implanted recipient observational study (Cochlear™ P-IROS). BMC Ear Nose Throat Disord. 2014, 14, 10. [Google Scholar] [CrossRef]
- Crawford, C.M.; Ramlackhan, K.; Singh, G.; Fenske, M.J. Subjective Impact of Age-Related Hearing Loss Is Worse for Those Who Routinely Experience Boredom and Failures of Attention. Ear Hear. 2023, 44, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Tyler, R.S. The Consumer Handbook on Tinnitus; Auricle Ink Publishers: Big Park, AZ, USA, 2008. [Google Scholar]
- Távora-Vieira, D.; Marino, R.; Krishnaswamy, J.; Kuthbutheen, J.; Rajan, G.P. Cochlear implantation for unilateral deafness with and without tinnitus: A case series. Laryngoscope 2013, 123, 1251–1255. [Google Scholar] [CrossRef]
- Assouly, K.K.S.; Arts, R.A.G.J.; Graham, P.L.; van Dijk, B.; James, C.J. Influence of tinnitus annoyance on hearing-related quality of life in cochlear implant recipients. Sci. Rep. 2022, 12, 14423. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, L.; Gilles, A.; Shekhawat, G.S. Hearing more to hear less: A scoping review of hearing aids for tinnitus relief. Int. J. Audiol. 2022, 61, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Tunkel, D.E.; Bauer, C.A.; Sun, G.H.; Rosenfeld, R.M. American Academy of Otolaryngology—Head and Neck Foundation Clinical Practice Guideline: Tinnitus. Otolaryngol. Neck Surg. 2014, 151, P20. [Google Scholar] [CrossRef]
- Tyler, R. Tinnitus Treatment and the Effectiveness Of Hearing Aids: Hearing Care Professional Perceptions. Hearing Review. 2008. Available online: https://hearingreview.com/practice-building/practice-management/tinnitus-treatment-and-the-effectiveness-of-hearing-aids-hearing-care-professional-perceptions (accessed on 1 August 2022).
- Miyamoto, R.T.; Wynne, M.K.; McKnight, C.; Bichey, B. Electrical Suppression of Tinnitus via Cochlear Implants. Int. Tinnitus J. 1997, 3, 35–38. [Google Scholar]
- Holmes, S.; Padgham, N.D. ‘‘Ringing in the Ears’’: Narrative Review of Tinnitus and Its Impact. Biol. Res. Nurs. 2011, 13, 97–108. [Google Scholar] [CrossRef]
- Folmer, R.L.; Martin, W.H.; Shi, Y. Tinnitus: Questions to reveal the cause, answers to provide relief. J. Fam. Pract. 2004, 53, 532–540. [Google Scholar]
- Holgers, K.-M.; Håkansson, B.E.V. Original Article: Sound stimulation via bone conduction for tinnitus relief: A pilot study: Estimulación Sonora por vía ósea para mejorar el acúfeno: Un estudio piloto. Int. J. Audiol. 2002, 41, 293–300. [Google Scholar] [CrossRef]
- Yang, Y.; Longworth, L.; Brazier, J. An assessment of validity and responsiveness of generic measures of health-related quality of life in hearing impairment. Qual. Life Res. 2013, 22, 2813–2828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dixon, P.R.; Feeny, D.; Tomlinson, G.; Cushing, S.L.; Chen, J.M. Improving the Hearing Status Discrimination of the Health Utilities Index, Mark 3: Design of the Hearing Status Classification System. Otol. Neurotol. 2022, 43, E1069–E1076. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, B.; Krog, N.H.; Kvestad, E.; Hoffman, H.J.; Tambs, K. Occupation and the risk of bothersome tinnitus: Results from a prospective cohort study (HUNT). BMJ Open 2012, 2, e000512. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R. Epidemiology of Tinnitus in Adults; Springer: New York, NY, USA, 2011; pp. 29–37. [Google Scholar] [CrossRef]


| Participant Characteristics, (n = 42) | n (%) |
|---|---|
| Age (years) mean ± SD (min–max) | 40.21 ± 14.58 (18–66) |
| Gender | |
| Female | 17 (40.5) |
| Male | 25 (59.5) |
| Country of residence | |
| Colombia | 36 (85.7) |
| Poland | 6 (14.3) |
| Tinnitus | |
| Presence of tinnitus pre implant | 23 (54.8) |
| No presence of tinnitus pre implant | 19 (45.2) |
| Tinnitus | Test | p-Value | Mean Pre-Implantation (Standard Deviation) | Mean Post-Implantation (Standard Deviation) | Mean Score Improvement (Standard Deviation) | Effect Size (d)/Cohen’s d | |
|---|---|---|---|---|---|---|---|
| Acoustic Implant: BCHA | |||||||
| HUI-3 Utility Score | Yes | WSRT | 0.218 | 0.624 (0.310) | 0.678 (0.269) | 0.054 (0.320) | 0.182 |
| No | WSRT | 0.277 | 0.811 (0.188) | 0.876 (0.158) | 0.065 (0.224) | 0.176 | |
| HUI-3 Hearing Attribute Score | Yes | WSRT | 0.139 | 0.865 (0.145) | 0.916 (0.067) | 0.051 (0.147) | 0.218 |
| No | WSRT | 0.053 | 0.857 (0.153) | 0.933 (0.096) | 0.076 (0.170) | 0.313 | |
| Global SSQ Score | Yes | Paired Samples t-test | 0.000 | 4.794 (1.863) | 6.566 (1.640) | 1.772 (1.920) | 0.923 |
| No | Paired Samples t-test | 0.000 | 5.036 (1.729) | 7.684 (1.200) | 2.648 (1.833) | 1.445 | |
| n | HUI-3 Pre-Implant r (p-Value) | Change in HUI-3 r (p-Value) | Hearing Attribute Pre-Implant r (p-Value) | Change in Hearing r (p-Value) | SSQ Score Pre-Implant r (p-Value) | Change in SSQ Score r (p-Value) | |
|---|---|---|---|---|---|---|---|
| Tinnitus status in BCHA patients | 42 | −0.343 (0.026) | −0.019 (0.906) | 0.027 (0.866) | −0.081 (0.612) | −0.069 (0.666) | −0.232 (0.141) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.; Lewis, A.T. Influence of Bone Conduction Hearing Device Implantation on Health-Related Quality of Life for Patients with and without Tinnitus. Audiol. Res. 2023, 13, 573-585. https://doi.org/10.3390/audiolres13040050
Khan N, Lewis AT. Influence of Bone Conduction Hearing Device Implantation on Health-Related Quality of Life for Patients with and without Tinnitus. Audiology Research. 2023; 13(4):573-585. https://doi.org/10.3390/audiolres13040050
Chicago/Turabian StyleKhan, Nasrene, and Aaran T. Lewis. 2023. "Influence of Bone Conduction Hearing Device Implantation on Health-Related Quality of Life for Patients with and without Tinnitus" Audiology Research 13, no. 4: 573-585. https://doi.org/10.3390/audiolres13040050
APA StyleKhan, N., & Lewis, A. T. (2023). Influence of Bone Conduction Hearing Device Implantation on Health-Related Quality of Life for Patients with and without Tinnitus. Audiology Research, 13(4), 573-585. https://doi.org/10.3390/audiolres13040050








