Prevalence of Congenital Infections in Newborns and Universal Neonatal Hearing Screening in Santa Catarina, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Study Site
2.2. Screening and Data-Collection Procedure
2.3. Outcome Variable
2.4. Main Exposure Variable and Covariates
2.5. Data Analysis
2.6. Ethical Aspects
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministério da Saúde. Available online: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt1459_24_06_2011.html (accessed on 20 December 2022).
- Klossoswski, D.G.; de Godói, V.C.; Xavier, C.R.; Fujinaga, C.I. Assistência integral ao recém-nascido prematuro: Implicações das práticas e da política pública. Rev. CEFAC 2016, 18, 137–150. [Google Scholar] [CrossRef]
- Bittencourt, S.D.D.A.; Vilela, M.E.D.A.; Marques, M.C.D.O.; dos Santos, A.M.; da Silva, C.K.R.T.; Domingues, R.M.S.M.; Reis, A.C.; Santos, G.L.D. Atenção ao parto e nascimento em Maternidades da Rede Cegonha/Brasil: Avaliação do grau de implantação das ações. Ciência Saúde Coletiva 2021, 26, 801–821. Available online: https://www.scielo.br/j/csc/a/4p3vFS9znjmjkKxrXBFdrMM/?format=pdf&lang=pt (accessed on 20 December 2022). [CrossRef]
- Neu, N.; Duchon, J.; Zachariah, P. TORCH Infections. Clin. Perinatol. 2015, 42, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.S.B.; Duarte, G.; Passos, M.R.L.; Sztajnbok DC das, N.; Menezes, M.L.B. Protocolo Brasileiro para Infecções Sexualmente Transmissíveis 2020: Sífilis congênita e criança exposta à sífilis. Epidemiol. Serviços Saúde 2021, 30, 1–10. Available online: https://www.scielo.br/j/ress/a/SwXRF6pXG3hX58K86jDSckv/?format=pdf&lang=es (accessed on 20 December 2022). [CrossRef]
- Ministério da Saúde. Pré-natal e Puerpério: Atenção Qualificada e Humanizada. 2006. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/manual_pre_natal_puerperio_3ed.pdf (accessed on 20 December 2022).
- Gontijo, M.G. Fatores de Risco Associados a Toxoplasmose Gestacional nas Unidade Básicas de Saúde dos Setores Vila Nova e Sevilha de Gurupi, Tocantins, Brasil. Rev. CEREUS 2014, 6, 145–157. Available online: http://ojs.unirg.edu.br/index.php/1/article/view/793 (accessed on 20 December 2022).
- Rocha, M.D.O.; Rocha, L.M.D.S.; Pimenta, M.P.D.C.; Caldeira, C.G.; Damas, D.P.; Pimentel, J.P.; de Aguiar, R.A.L.P.; Quintino, N.D.; Cardoso, C.S. Tendência temporal e perfil da mortalidade infantil por malformação congênita em uma região de saúde de Minas Gerais. Rev. Eletrônica Acervo Saúde 2021, 13, e6808. [Google Scholar] [CrossRef]
- Lopes, M.K.D.; Santos, T.M.M. Comparison of Indicators of Risk of Deafness in Newborns Studied in the Years 1995 and 2005. Int. Arch. Otorhinolaryngol. 2011, 15, 35–40. Available online: http://www.arquivosdeorl.org.br/conteudo/acervo_port.asp?id=738 (accessed on 20 December 2022).
- Queiroz, K.M.P.; Paredes, H.D.M.T.; Costa, A.C.S.; Silva, M.O.C.; Costa, F.V.; Lima, L.A.V.; Carmo, C.N.; Capelli, J.C.S.; Correa, V.O.S. Infecções congênitas em um hospital público de referência em Macaé, Rio de Janeiro, no biênio 2016–2017. Rev. Saúde Pública Paraná 2021, 4, 29–43. [Google Scholar] [CrossRef]
- da Saúde, M. Boletim Epidemiológico de Sífilis–2018|Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Available online: http://www.aids.gov.br/pt-br/pub/2018/boletim-epidemiologico-de-sifilis-2018 (accessed on 20 December 2022).
- da Saúde, M. Boletim Epidemiológico de Sífilis 2021|Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Available online: http://www.aids.gov.br/pt-br/pub/2021/boletim-epidemiologico-de-sifilis-2021 (accessed on 20 December 2022).
- da Saúde, M. Boletim Epidemiológico HIV/Aids 2018|Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Available online: http://www.aids.gov.br/pt-br/pub/2018/boletim-epidemiologico-hivaids-2018 (accessed on 20 December 2022).
- da Saúde, M. Boletim Epidemiológico HIV/Aids 2021|Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Available online: http://www.aids.gov.br/pt-br/pub/2021/boletim-epidemiologico-hivaids-2021 (accessed on 20 December 2022).
- Departamento Científico de Neonatologia. Toxoplasmose congênita Documento Científico. Available online: https://www.sbp.com.br/fileadmin/user_upload/22620c-DC_-_Toxoplasmose_congenita.pdf (accessed on 20 December 2022).
- Marin, L.J.; Santos de Carvalho Cardoso, E.; Bispo Sousa, S.M.; Debortoli de Carvalho, L.; Marques Filho, M.F.; Raiol, M.R.; Gadelha, S.R. Prevalence and clinical aspects of CMV congenital Infection in a low-income population. Virol. J. 2016, 13, 148. [Google Scholar] [CrossRef]
- Chuang, C.Á.; Ramos, H.H.; Zelada, B.Ú.; López, C.M.T.; Villavicencio, L.L.; Peret, L.M.; Gonzalez Munoz, C.; Barria Espinoza, T.; Izquierdo Copiz, G. Cribado de infección por citomegalovirus congénito en recién nacidos de alto riesgo. Rev. Chil. Infectología 2021, 38, 45–53. Available online: https://www.scielo.cl/scielo.php?pid=S071610182021000100045&script=sci_arttext (accessed on 20 December 2022). [CrossRef]
- Moraes, M.M.D.; Cruz, A.C.R.; Silva, D.D.F.L.D.; Sagica, F.D.E.S.; Santos, E.C.D.O. Trajetória da rubéola no Estado do Pará, Brasil: Rumo à erradicação. Rev. Pan-Amaz. Saúde 2015, 6, 11–20. [Google Scholar] [CrossRef]
- Joint Committee on Infant Hearing. Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. Pediatrics 2007, 120, 898–921. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Infant Hearing. Year 2019 Position statement: Principles and guidelines for early hearing detection and intervention programs. Jt. Comm. Infant Hear. 2019, 4, 1–44. [Google Scholar]
- Lewis, D.; Antonio, S.; Marone, M.; Mendes, B.; Laercio, O.; Cruz, M.; Nóbrega, M. Multiprofessional committee on auditory health -COMUSA Summary. Braz. J. Otorhinolaryngol. 2010, 76. Available online: https://www.scielo.br/j/bjorl/a/6Ffk6pTDGccSf4NWFTXy5zH/?lang=en&format=pdf (accessed on 20 December 2022).
- da Saúde, M. Diretrizes de Atenção da Triagem Auditiva Neonatal. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_atencao_triagem_auditiva_neonatal.pdf (accessed on 20 December 2022).
- Januário, G.C.; Lemos, S.M.A.; de Lima Friche, A.A.; Alves, C.R.L. Quality indicators in a newborn hearing screening service. Braz. J. Otorhinolaryngol. 2015, 81, 255–263. [Google Scholar] [CrossRef]
- Vernier, L.S.; Cazella, S.C.; Levandowski, D.C. Triagem Auditiva Neonatal: Protocolos, obstáculos e perspectivas de fonoaudiólogos no Brasil-10 anos da Lei Federal Brasileira 12.303/2010. CoDAS 2020, 34, e20200331. [Google Scholar] [CrossRef]
- Ribeiro, G.E.; Weber, S.A.T.; da Silva, D.P.C. Territorial distribution and quality indicators of compulsory Neonatal Hearing Screening in Brazil after Law 12,303/2010. Rev. CEFAC 2020, 22, e7919. [Google Scholar] [CrossRef]
- de Avila, A.T.V.; Teixeira, A.R.; Vernier, L.S.; Silveira, A.L. Universal neonatal hearing screening program at a university hospital: An analysis using quality indicators. Rev. CEFAC 2021, 23, e4421. [Google Scholar] [CrossRef]
- Weinstein, M.C.A.; Durante, A.S. Triagem auditiva em neonatos. In Lopes of Novo Tratado de Fonoaudiologia; Manole: Barueri, Brazil, 2011; pp. 145–148. [Google Scholar]
- da Saúde, M. Decreto nº 7.612, de 17 de novembro de 2011; Institui o Plano Nacional dos Direitos da Pessoa com Deficiência–Plano Viver sem Limite, Presidência da República, Casa Civil: Brasília, Braxil, 2011. [Google Scholar]
- de Mattos, W.M.; Cardoso, L.F.; Bissani, C.; Pinheiro, M.M.C.; Viveiros, C.M.; Carreirão Filho, W. Análise da implantação de programa de triagem auditiva neonatal em um hospital universitário. Rev. Bras. Otorrinolaringol. 2009, 75, 237–244. [Google Scholar] [CrossRef]
- Kemp, A.A.T.; Delecrode, C.R.; da Silva, G.C.; Martins, F.; Frizzo, A.C.F.; Cardoso, A.C.V. Neonatal hearing screening in a low-risk maternity hospital in São Paulo state. Braz. J. Otorhinolaryngol. 2015, 81, 505–513. [Google Scholar] [CrossRef]
- Onoda, R.M.; de Azevedo, M.F.; dos Santos, A.M.N. Triagem auditiva neonatal: Ocorrência de falhas, perdas auditivas e indicadores de riscos. Braz. J. Otorhinolaryngol. 2011, 77, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, H.G.; de Melo, L.P.F.; Buarque, L.F.S.F.; Guerra, R.O. Overview of newborn hearing screening programs in Brazilian maternity hospitals. Braz. J. Otorhinolaryngol. 2014, 80, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.C.M.P.; Rossi, T.R.D.F.; Françozo, M.D.F.D.C.; Collela-Santos, M.F.; Correa, C.R. Analysis of neonatal hearing screening program performed on an outpatient basis: Analysis of an outpatient hearing screening program. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Sabbag, J.C.; de Lacerda, A.B.M. Rastreamento e monitoramento da Triagem Auditiva Neonatal em Unidade de Estratégia de Saúde da Família: Estudo-piloto. CoDAS 2017, 29, e20160102. Available online: https://www.scielo.br/pdf/codas/v29n4/en_2317-1782-codas-29-4-e20160102.pdf (accessed on 20 December 2022). [CrossRef] [PubMed]
- Galvão, M.B.; Fichino, S.N.; Lewis, D.R. Processo do diagnóstico audiológico de bebês após a falha na triagem auditiva neonatal. Distúrbios Comun. 2021, 33, 416–427. [Google Scholar] [CrossRef]
- Comitê Multiprofissional em Saúde Auditiva. Triagem Auditiva Neonatal Universal em Tempos de Pandemia. 2020. Available online: https://www.sbfa.org.br/portal2017/pdf/cvd19-nota-tecnica-comusa.pdf (accessed on 20 December 2022).
- Didoné, D.D.; Garcia, M.V.; Kunst, L.R.; Vieira, E.P.; da Silveira, A.F. Correlação dos indicadores de risco para deficiência auditiva com a “Falha” na triagem auditiva neonatal. Saúde 2013, 39, 113–120. [Google Scholar] [CrossRef]
- Botasso, K.D.C.; Lima, M.C.P.M.; Correa, C.R.S. Association between failure in otoacoustic emissions and risk indicator for hearing loss. Rev. CEFAC 2021, 23, e10620. [Google Scholar] [CrossRef]
- Vanassi, B.M.; Parma, G.C.; Magalhaes, V.S.; dos Santos, A.C.C.; Iser, B.P.M. Congenital anomalies in Santa Catarina: Case distribution and trends in 2010–2018. Rev. Paul. Pediatr. 2022, 40, e2020331. [Google Scholar] [CrossRef]
- Pereira, P.K.S.; Martins, A.D.S.; Vieira, M.R.; de Azevedo, M.F. Programa de triagem auditiva neonatal: Associação entre perda auditiva e fatores de risco. Pró-Fono Rev. Atualização Científica 2007, 19, 267–278. [Google Scholar] [CrossRef]
- Choi, K.Y.; Lee, B.S.; Choi, H.G.; Park, S.K. Analysis of the Risk Factors Associated with Hearing Loss of Infants Admitted to a Neonatal Intensive Care Unit: A 13-Year Experience in a University Hospital in Korea. Int. J. Environ. Res. Public Health 2020, 17, 8082. [Google Scholar] [CrossRef]
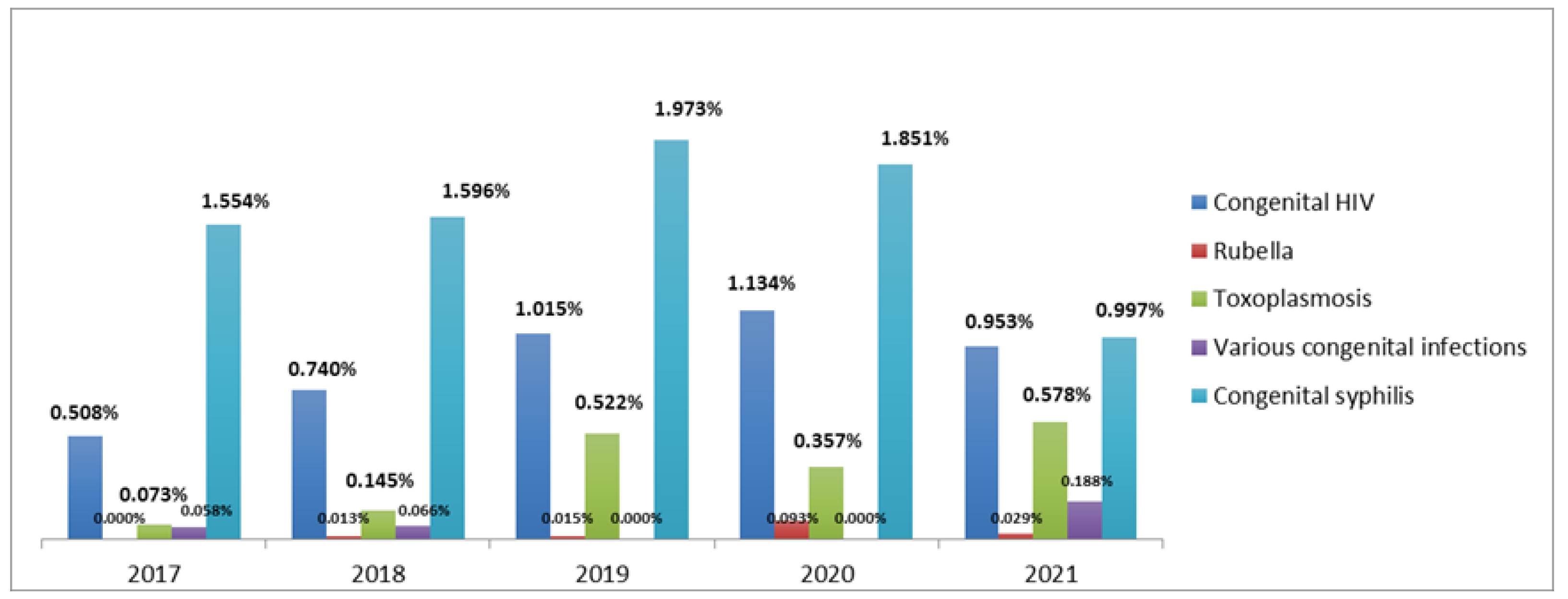
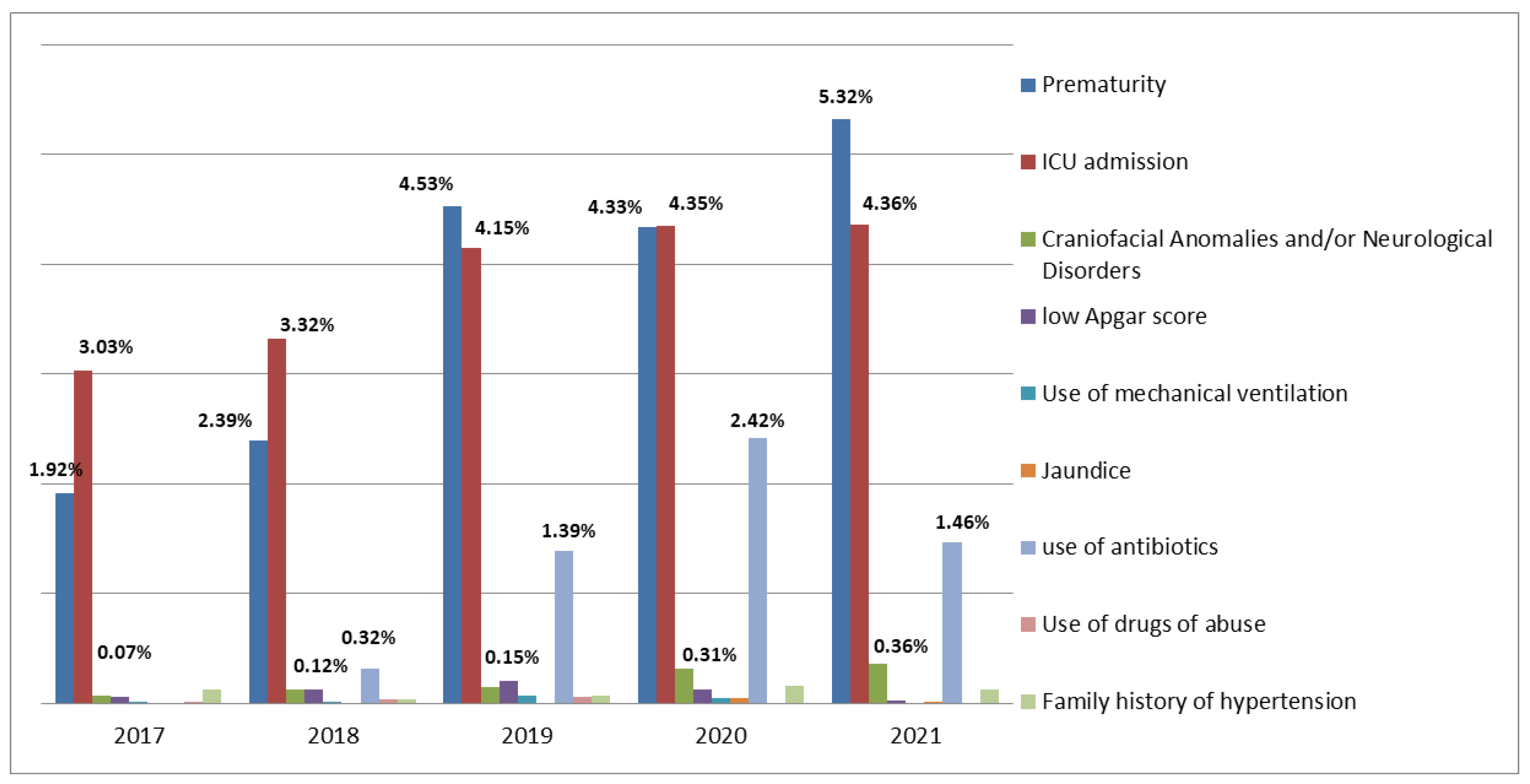
| Year | Data from the SC Department of Health | Data from DATASUS | Data from the SC Department of Health | Data from DATASUS | Data from the SC Department of Health | Data from DATASUS |
|---|---|---|---|---|---|---|
| Health Facility | MCD * Florianópolis, SC | HRSJ ** São José, SC | Percentage of Otovida Coverage—Overall | Percentage of Otovida Coverage—Overall | ||
| 2017 | 98.01% | 98.43% | 89.48% | 87.46% | 93.82% | 92.97% |
| 2018 | 109.38% | 110.24% | 89.16% | 90.28% | 99.36% | 100.37% |
| 2019 | 101.96% | 102.48% | 106.44% | 105.92% | 104.19% | 104.20% |
| 2020 | 97.04% | 97.46% | 100.37% | 105.55% | 98.72% | 101.46% |
| 2021 | 110.35% | 114.01% | 100.23% | 101.90% | 105.02% | 107.58% |
| Total | 103.35% | 104.42% | 96.79% | 97.72% | 100.56% | 101.05% |
| Annual average | 103.35% | 104.52% | 97.13% | 98.22% | 100.22% | 101.32% |
| Overall annual average | 100.77% | |||||
| Variable | Year of Birth | n | % | 95% CI | p-Value * | p-Value ** | p-Value *** |
|---|---|---|---|---|---|---|---|
| Craniofacial Anomalies | 2017 | 5 | 0.0726 | 0.0236 to 0.169 | 0.1113 | 0.0148 | 0.1877 |
| 2018 | 8 | 0.106 | 0.0456 to 0.208 | ||||
| 2019 | 10 | 0.145 | 0.0696 to 0.267 | ||||
| 2020 | 15 | 0.233 | 0.130 to 0.384 | ||||
| 2021 | 13 | 0.188 | 0.100 to 0.321 | ||||
| Total | 51 | 0.147 | 0.109 to 0.193 | ||||
| Neurological Disorders | 2017 | 0 | 0 | 0.000 to 0.0535 | <0.0001 | <0.0001 | 0.0057 |
| 2018 | 1 | 0.0132 | 0.000334 to 0.0736 | ||||
| 2019 | 0 | 0 | 0.000 to 0.0535 | ||||
| 2020 | 5 | 0.0776 | 0.0252 to 0.181 | ||||
| 2021 | 12 | 0.173 | 0.0896 to 0.303 | ||||
| Total | 18 | 0.0519 | 0.0307 to 0.0819 |
| Variable | Adjusted OR | 95% CI | p Value |
|---|---|---|---|
| Congenital HIV | 3.8848 to 11.4375 | ||
| No | 1.0000 | <0.0001 | |
| Yes | 6.6658 | ||
| Congenital syphilis | |||
| No | 1.0000 | 1.4465 to 3.9024 | 0.0006 |
| Yes | 2.3759 | ||
| Craniofacial Anomalies and/or Neurological Disorders | |||
| No | 1.0000 | 21.9068 to 89.2331 | <0.0001 |
| Yes | 44.2133 | ||
| Admission to the ICU | |||
| No | 1.0000 | 2.1486 to 4.5434 | <0.0001 |
| Yes | 3.1244 | ||
| Prematurity | |||
| No | 1.0000 | 3.6051 to 6.9432 | <0.0001 |
| Yes | 5.0031 | ||
| Antibiotic use | |||
| No | 1.0000 | 1.0092 to 4.9801 | 0.0474 |
| Yes | 2.2419 | ||
| Year of Birth | |||
| 2017 | 1.0000 | ||
| 2018 | 1.1525 | 0.8585 to 1.5472 | 0.3449 |
| 2019 | 1.7179 | 1.3017 to 2.2671 | 0.0001 |
| 2020 | 0.3440 | 0.2251 to 0.5257 | <0.0001 |
| 2021 | 0.0900 | 0.0449 to 0.1803 | <0.0001 |
| Maternal age | |||
| X | 1.0000 | 0.9672 to 0.9981 | 0.0279 |
| x + 1 year | 0.9825 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besen, E.; Paiva, K.M.; Cigana, L.B.; Machado, M.J.; Samelli, A.G.; Haas, P. Prevalence of Congenital Infections in Newborns and Universal Neonatal Hearing Screening in Santa Catarina, Brazil. Audiol. Res. 2023, 13, 107-115. https://doi.org/10.3390/audiolres13010011
Besen E, Paiva KM, Cigana LB, Machado MJ, Samelli AG, Haas P. Prevalence of Congenital Infections in Newborns and Universal Neonatal Hearing Screening in Santa Catarina, Brazil. Audiology Research. 2023; 13(1):107-115. https://doi.org/10.3390/audiolres13010011
Chicago/Turabian StyleBesen, Eduarda, Karina Mary Paiva, Luciana Berwanger Cigana, Marcos José Machado, Alessandra Giannella Samelli, and Patrícia Haas. 2023. "Prevalence of Congenital Infections in Newborns and Universal Neonatal Hearing Screening in Santa Catarina, Brazil" Audiology Research 13, no. 1: 107-115. https://doi.org/10.3390/audiolres13010011
APA StyleBesen, E., Paiva, K. M., Cigana, L. B., Machado, M. J., Samelli, A. G., & Haas, P. (2023). Prevalence of Congenital Infections in Newborns and Universal Neonatal Hearing Screening in Santa Catarina, Brazil. Audiology Research, 13(1), 107-115. https://doi.org/10.3390/audiolres13010011








