Rehabilitation of Severe-to-Profound Hearing Loss in Adults in Sweden
Abstract
:1. Introduction
- (1)
- To present data on variables that could influence the outcomes of audiological rehabilitation.
- (2)
- To evaluate the influence of the type of HL (SNHL/MHL) and speech recognition on the outcomes of audiological rehabilitation.
- (3)
- To compare the outcomes of audiological rehabilitation over time between HA and CI users.
2. Materials and Methods
2.1. The Swedish Registry for Adult Patients with STPHL
2.2. General and Baseline Questionnaire
2.3. Follow-Up Questionnaire
2.4. Statistical Analysis
3. Results
3.1. General and Baseline Questionnaire
| HA (%) | CI (%) | Extended Audiological Rehabilitation (%) | Reference | ||
|---|---|---|---|---|---|
| Total | 87 | 10 | 38 | [16] | |
| Sex | Female | 86 | 12 | 43 | [16] |
| Male | 88 | 9 | 34 | ||
| Age at registration, years | 19–40 | 78 | 11 | 44 | [16] |
| 41–60 | 83 | 17 | 53 | ||
| 61–80 | 88 | 12 | 44 | ||
| ≥81 | 92 | 4 | 20 | ||
| Education level | Elementary school | 90 | 7 | 34 | [16] |
| Secondary school | 86 | 13 | 41 | ||
| Vocational school | 81 | 6 | 45 | ||
| Folk high school | 90 | 9 | 42 | ||
| College | 84 | 17 | 48 | ||
| Other education | 86 | 11 | 34 | ||
| Degree of hearing loss, dB | >100 | 67 | 24 | 43 | [16] |
| 91–100 | 86 | 16 | 53 | ||
| 81–90 | 91 | 11 | 41 | ||
| 70–80 | 94 | 2 | 29 | ||
| Deaf blindness | Dual sensory loss | 89 | 8 | 32 | [23] |
| STPHL | 86 | 12 | 40 |
|
HADS Anxiety ≥ 8 (%) |
HADS Depression ≥ 8 (%) |
ES > 70 (%) | Reference | ||
|---|---|---|---|---|---|
| Onset of HL | <3 years | 31 | 22 | 40 | [3] |
| ≥3 years | 30 | 24 | 25 | ||
| Tinnitus | Often, always | 54 | 37 | 55 | [3] |
| Sometimes, never | 26 | 17 | 38 | ||
| Vertigo | Often, always | 59 | 45 | 55 | [3] |
| Sometimes, never | 33 | 21 | 42 | ||
| Cochlear implant | Yes | 37 | 18 | 30 | [3] |
| No | 30 | 23 | 40 | ||
| Deaf blindness | Dual sensory loss | 41 | 34 | 50 | [23] |
| STPHL | 29 | 19 | 36 |
| MHL n = 664 | SNHL n = 3450 | |
|---|---|---|
| Sex, n, % | ||
| Men | 299 (45%) | 1769 (51%) |
| Women | 365 (55%) | 1681 (49%) |
| Age classes (years), n, % | ||
| 19–40 | 17 (3%) | 348 (10%) |
| 41–60 | 86 (13%) | 657 (19%) |
| 61–80 | 349 (53%) | 1417 (41%) |
| 81–100 | 212 (32%) | 1028 (30%) |
| Education, n, % | ||
| Elementary school | 294 (45%) | 1296 (38%) |
| Training school | 34 (5%) | 201 (6%) |
| High school | 150 (23%) | 1035 (30%) |
| Other education | 84 (13%) | 363 (11%) |
| University | 97 (15%) | 545 (16%) |
| HA | CI | Extended Rehabilitation | |
|---|---|---|---|
| MHL, % | 95 | 4 | 45 |
| SNHL, % | 89 | 12 | 45 |
| Adjusted OR (95% confidence interval), p-value | 2.23 a (1.52–3.27) p < 0.001 | 0.32 b (0.21–0.49) p < 0.001 | 1.02 c (0.85–1.23) ns |
| Speech recognition≤50%, (%) | 92 | 16 | 58 |
| Speech recognition >50%, (%) | 97 | 2 | 47 |
| Adjusted OR (95% confidence interval), p-value | 0.40 d (0.26–0.61) p < 0.001 | 7.41 e (4.69–11.69) p < 0.001 | 1.55 f (1.27–1.90) p < 0.001 |
| HADS Anxiety ≥ 8 | HADS Depression ≥ 8 | ES ≥ 70 | |
|---|---|---|---|
| MHL, % | 32 | 25 | 42 |
| SNHL, % | 31 | 22 | 39 |
| Adjusted OR (95% (confidence interval), p-value | 1.18 a (0.82–1.71) p = ns | 1.26 b (0.84–1.88) p = ns | 1.05 c (0.88–1.27) p = ns |
| Speech recognition≤50%, (%) | 29 | 22 | 43 |
| Speech recognition >50%, (%) | 27 | 19 | 38 |
| Adjusted OR (95% (confidence interval), p-value | 1.13 d (0.75–1.70) p = ns | 1.26 e (0.81–1.98) p = ns | 1.23 f (1.02–1.50) p = ns |
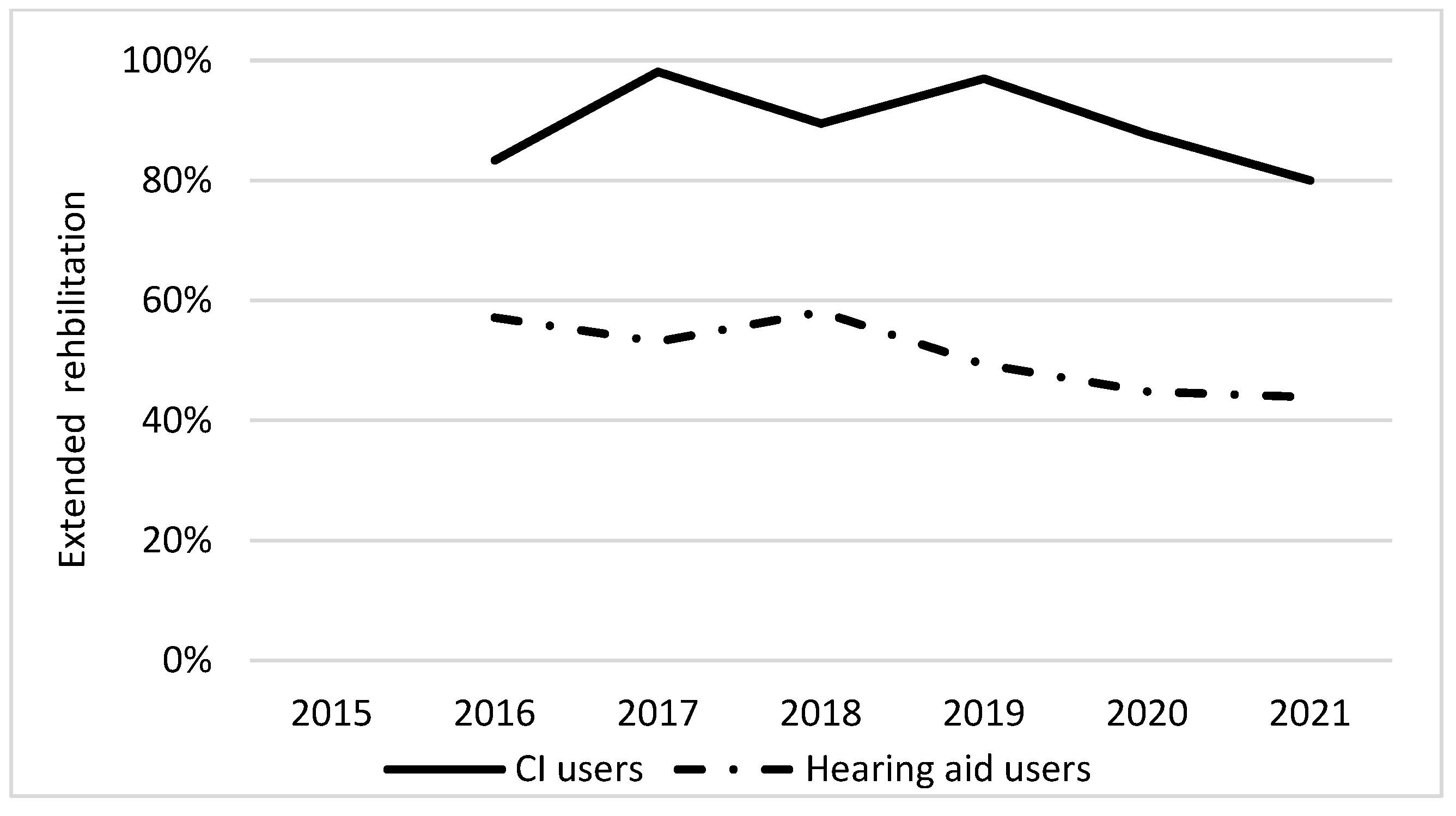
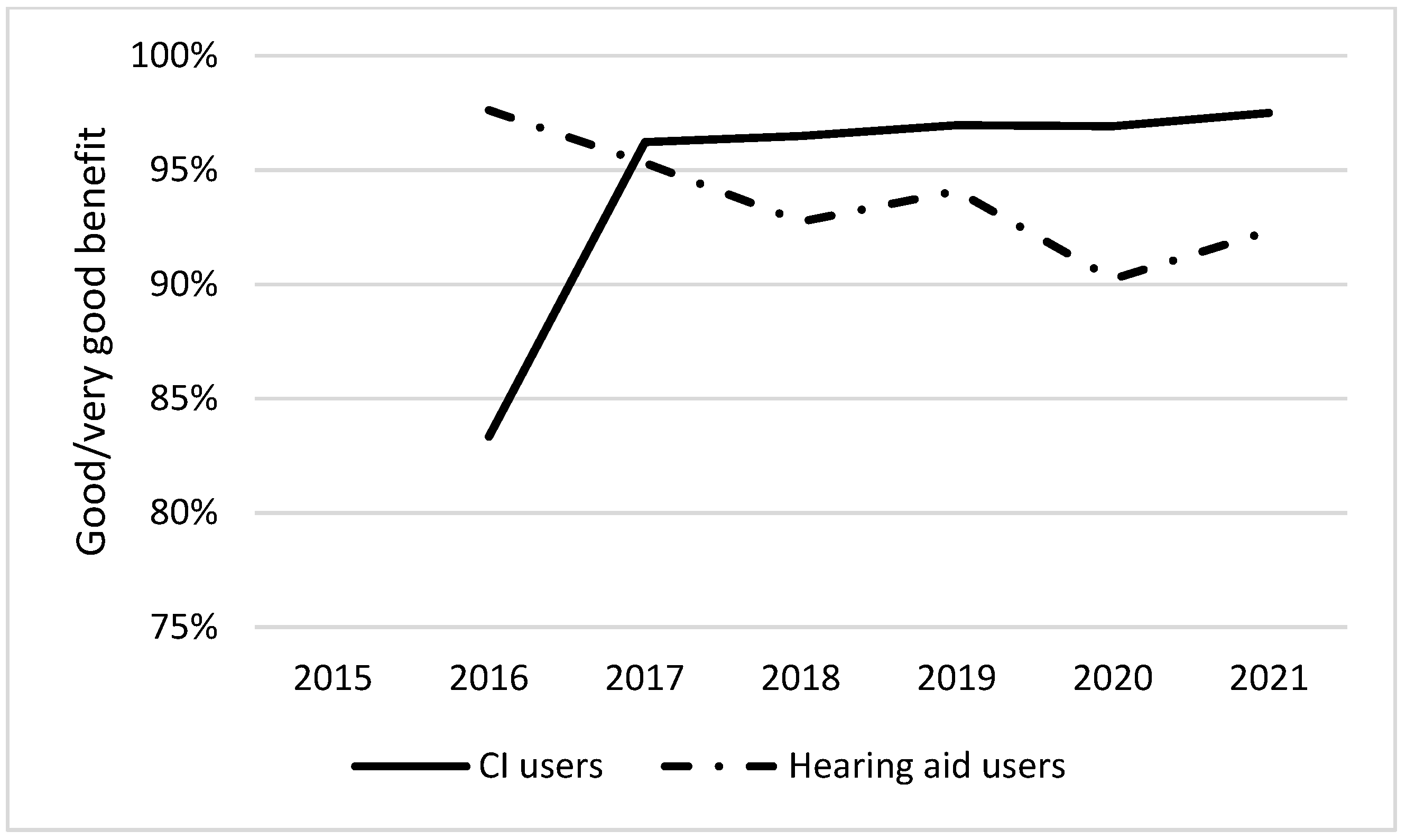
3.2. Follow-Up Questionnaire
| Extended Audiological Rehabilitation | Good/Very Good Benefit of Rehabilitation | Good/Very Good Benefit of HA/CI | |
|---|---|---|---|
| STPHL, total, % | 52 a | 93 b | 90 c |
| MHL, % | 43 | 97 | 96 |
| SNHL, % | 53 | 93 | 90 |
| Adjusted OR (95% confidence interval), p-value | 0.67 (0.54–0.83) p < 0.001 | 2.78 (1.40–5.52) p < 0.003 | 2.58 (1.48–5.50) p < 0.001 |
| Speech recognition, total, % | 50 d | 94 e | 91 f |
| Speech recognition ≤50%, (%) | 53 | 93 | 87 |
| Speech recognition >50%, (%) | 45 | 95 | 96 |
| Adjusted OR (95% confidence interval), p-value | 1.48 (1.23–1.78) p < 0.001 | 0.64 (0.41–0.98) p = ns | 0.28 (0.18–0.43) p < 0.001 |
| Medical | Hearing | Patient | Communi-cation | CI Invest Start | Unknown | |
|---|---|---|---|---|---|---|
| Baseline | (%) | (%) | (%) | (%) | (%) | (%) |
| STPHL, total a | 44 | 36 | 17 | 2 | 14 | 28 |
| Follow-up | (%) | (%) | (%) | (%) | (%) | (%) |
| STPHL, total b | 5 | 39 | 23 | 3 | 17 | 13 |
| MHLc | 8 | 56 | 15 | 0 | 8 | 13 |
| SNHLd | 5 | 37 | 25 | 4 | 18 | 12 |
| Speech recognition ≤50%e | 5 | 38 | 23 | 2 | 19 | 13 |
| Speech recognition >50%f | 5 | 42 | 23 | 3 | 14 | 15 |
4. Discussion
4.1. General and Baseline Questionnaire
4.2. Follow-Up Questionnaire
4.3. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciorba, A.; Guidi, M.P.; Skarżyński, P.H.; Bianchini, C.; Rosignoli, M.; Mazzoli, M.; Pelucchi, S.; Hatzopoulos, S. Rehabilitation of severe to profound sensorineural hearing loss in adults: Audiological outcomes. Ear Nose Throat J. 2021, 100, 215S–219S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringdahl, A.; Grimby, A. Severe-profound hearing impairment and health-related quality of life among post-lingual deafened Swedish adults. Scand. Audiol. 2000, 29, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.-I.; Hjaldahl, J.; Magnuson, A.; Ternevall, E.; Edén, M.; Skagerstrand, Å.; Jönsson, R. Severe to profound hearing impairment: Quality of life, psychosocial consequences and audiological rehabilitation. Disabil. Rehabil. 2015, 37, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Hallam, R.; Ashton, P.; Sherbourne, K.; Gailey, L. Acquired profound hearing loss: Mental health and other characteristics of a large sample. Int. J. Audiol. 2009, 45, 715–723. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Hearing; World Health Organization: Geneva, Switzerland, 2021.
- British Society of Audiology. Recommended Procedure. Pure-Tone Conduction and Bone-Threshold Audiometry with an without Masking. Available online: http://www.thebsa.org.uk/wp-content/uploads/2014/04/BSA_RP_PTA_FINAL_24Sept11_MinorAmend06Feb12.pdf (accessed on 28 July 2022).
- Löfvenberg, C.; Carlsson, P.-I.; Barrenäs, M.L.; Imic, D.; Carlsson, J.; Wigdén, J.; Westman, E. Prevalence of severe-to-Profound hearing loss in the adult Swedish population and comparison with cochlear implantation rate. Acta Otolaryngol. 2022, 142, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.W. Hearing aids and otosclerosis. Otolaryngol. Clin. North. Am. 1993, 26, 491–502. [Google Scholar] [CrossRef]
- Skarzynski, P.H.; Ciesla, K.; Lorens, A.; Wojcik, J.; Skarzynski, H. Cost-utility analysis of bilateral cochlear implantation in adults with severe to profound sensorineural hearing loss in Poland. Otol. Neurotol. 2021, 42, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, T.A.; Picou, E.M.; Shehorn, J.; Dittberner, A.B. Degree of hearing loss affects bilateral hearing aid benefits in ecologically relevant laboratory conditions. J. Speech Lang. Hear. Res. 2019, 62, 3834–3850. [Google Scholar] [CrossRef] [PubMed]
- Turton, L.; Souza, P.; Thibodeau, L.; Hickson, L.; Gifford, R.; Bird, J.; Stropahl, M.; Gailey, L.; Fulton, B.; Scarinci, N.; et al. Guidelines for best practice in the audiological management of adults with severe and profound hearing loss. Semin. Hear. 2020, 41, 141–246. [Google Scholar] [CrossRef] [PubMed]
- Gaylor, J.M.; Raman, G.; Chung, M.; Lee, J.; Rao, M.; Lau, J.; Poe, D.S. Cochlear implantation in adults: A systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Dwyer-Hemmings, L.; Manjaly, J.G.; Nash, R.; Mukherjee, A.; Lavy, J.A. Stapes surgery for profound hearing loss secondary to otosclerosis. Ear Nose Throat J. 2019, 98, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorkin, D.L. Cochlear implantation in the world’s largest medical device market: Utilization and awareness of cochlear implants in the United States. Cochlear Implants Int. 2013, 14, S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Turunen-Taheri, S.K.; Edén, M.; Hellström, S.; Carlsson, P.I. Rehabilitation of adult patients with severe-to-profound hearing impairment–why not cochlear implants? Acta Otolaryngol. 2019, 139, 604–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjaldahl, J.; Widén, S.; Carlsson, P. Severe to profound hearing impairment: Factors associated with the use of hearing aids and cochlear implants and participation in extended audiological rehabilitation. Hear. Balanc. Commun. 2016, 15, 6–15. [Google Scholar] [CrossRef]
- Swedish Quality Registry of Otorhinolaryngology. Available online: http://www.entqualitysweden.se (accessed on 27 July 2022).
- Turunen-Taheri, S.; Carlsson, P.-I.; Johnson, A.-C.; Hellström, S. Severe-to-profound hearing impairment: Demographic data, gender differences and benefits of audiological rehabilitation. Disabil. Rehabil. 2019, 41, 2766–2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisspers, J.; Nygren, A.; Söderman, E. Hospital Anxiety and Depression Scale (HAD): Some psychometric data for a Swedish sample. Acta Psychiatr. Scand. 1997, 96, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.M.; Alexander, G.C. The International Outcome Inventory for Hearing Aids (IOI-HA): Psychometric properties of the English version: El Inventario International de Resultados para Auxiliares Auditivos (IOI-HA): Propiedades psicometricas de la version en ingles. Int. J. Audiol. 2009, 41, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Turunen-Taheri, S.; Skagerstrand, Å.; Hellström, S.; Carlsson, P.I. Patients with severe-to-profound hearing impairment and simultaneous severe vision impairment: A quality-of-life study. Acta Otolaryngol. 2017, 137, 279–285. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löfvenberg, C.; Turunen-Taheri, S.; Carlsson, P.-I.; Skagerstrand, Å. Rehabilitation of Severe-to-Profound Hearing Loss in Adults in Sweden. Audiol. Res. 2022, 12, 433-444. https://doi.org/10.3390/audiolres12040044
Löfvenberg C, Turunen-Taheri S, Carlsson P-I, Skagerstrand Å. Rehabilitation of Severe-to-Profound Hearing Loss in Adults in Sweden. Audiology Research. 2022; 12(4):433-444. https://doi.org/10.3390/audiolres12040044
Chicago/Turabian StyleLöfvenberg, Christian, Satu Turunen-Taheri, Per-Inge Carlsson, and Åsa Skagerstrand. 2022. "Rehabilitation of Severe-to-Profound Hearing Loss in Adults in Sweden" Audiology Research 12, no. 4: 433-444. https://doi.org/10.3390/audiolres12040044
APA StyleLöfvenberg, C., Turunen-Taheri, S., Carlsson, P.-I., & Skagerstrand, Å. (2022). Rehabilitation of Severe-to-Profound Hearing Loss in Adults in Sweden. Audiology Research, 12(4), 433-444. https://doi.org/10.3390/audiolres12040044






