Perception Mechanism of Bone-Conducted Ultrasound and Its Clinical Use
Abstract
1. Introduction
2. Characteristics of Ultrasonic Perception
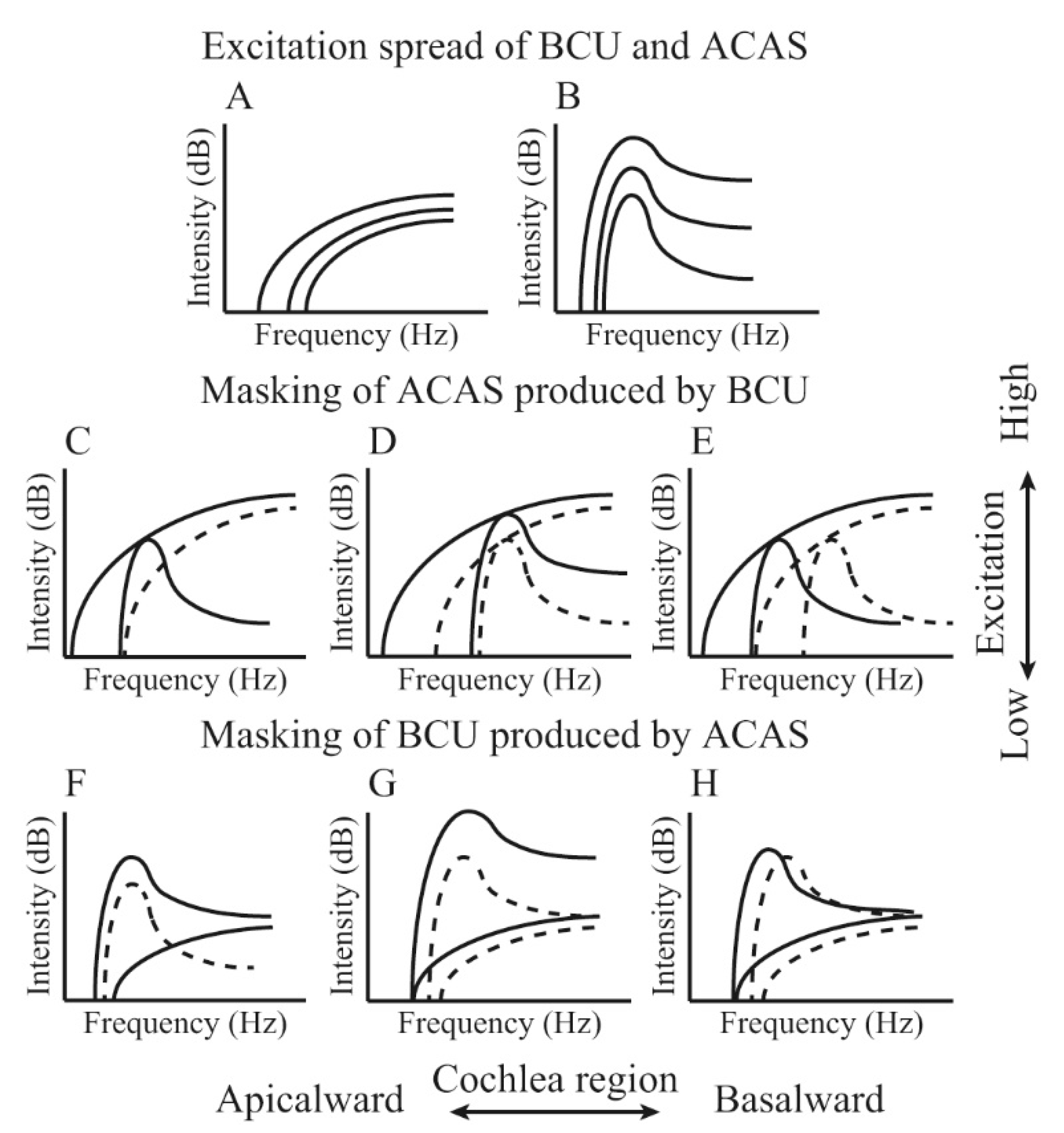
3. Peripheral Perception Mechanism of BCU
4. Ultrasonic Perception at the Central Level
5. Clinical Use of Ultrasonic Perception
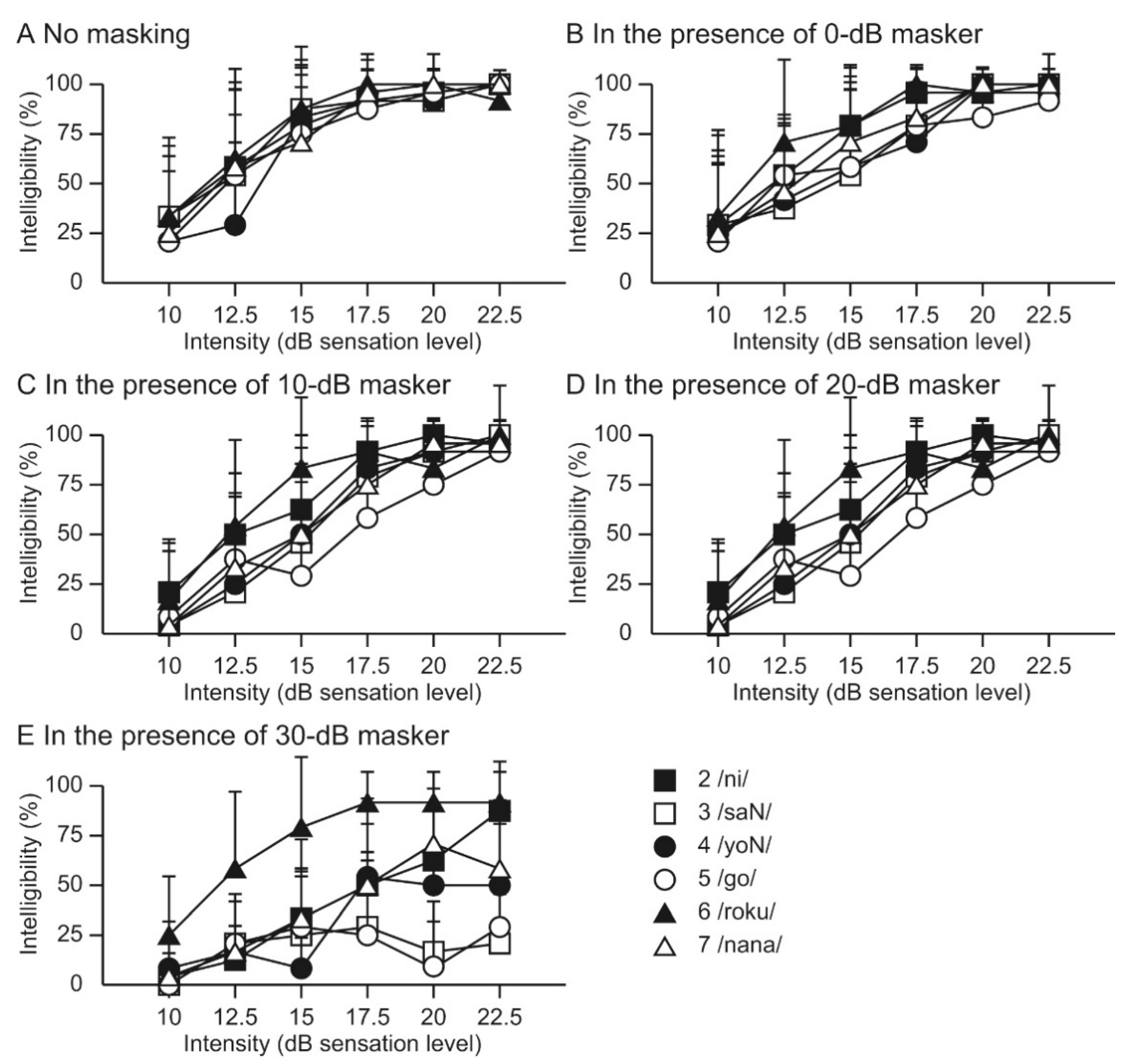
6. Recognition of Speech-Modulated BCU in Normal-Hearing Individuals
7. Recognition of Speech-Modulated BCU in Hearing-Impaired Patients
8. Application of BCU for Tinnitus Treatment
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wegel, R.P. Physical data and physiology of excitation of the auditory nerve. Anns. Otol. Rhinol. Lar. 1932, 41, 740–799. [Google Scholar] [CrossRef]
- Gavreau, V. Audibillite de sons de frequence elevee. Compt. Rendu. 1948, 226, 2053–2054. [Google Scholar]
- Pumphrey, R. Upper limit of frequency for human hearing. Nature 1950, 166, 571. [Google Scholar] [CrossRef] [PubMed]
- Dieroff, H.G.; Ertel, H. Some thoughts on the perception of ultrasonics by man. Arch. Otorhinolaryngol. 1975, 209, 277–299. [Google Scholar] [CrossRef]
- Corso, J.F. Bone-conduction thresholds for sonic and ultrasonic frequencies. J. Acoust. Soc. Am. 1963, 35, 1738–1743. [Google Scholar] [CrossRef]
- Haeff, A.V.; Knox, C. Perception of ultrasound. Science 1963, 139, 590–592. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakagawa, S.; Sakaguchi, T.; Hosoi, H. Ultrasonic masker clarifies ultrasonic perception in man. Hear. Res. 2003, 175, 171–177. [Google Scholar] [CrossRef]
- Lenhardt, M.L.; Skellett, R.; Wang, P.; Clarke, A.M. Human ultrasonic speech perception. Science 1991, 253, 82–85. [Google Scholar] [CrossRef]
- Hosoi, H.; Imaizumi, S.; Sakaguchi, T.; Tonoike, M.; Murata, K. Activation of the auditory cortex by ultrasound. Lancet 1998, 351, 496–497. [Google Scholar] [CrossRef]
- Imaizumi, S.; Hosoi, H.; Sakaguchi, T.; Watanabe, Y.; Sadato, N.; Nakamura, S.; Waki, A.; Yonekura, Y. Ultrasound activates the auditory cortex of profound deaf subjects. NeuroReport 2001, 12, 583–586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carlson, M.L. Cochlear Implantation in Adults. N. Engl. J. Med. 2020, 382, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Lieu, J.E.C.; Kenna, M.; Anne, S.; Davidson, L. Hearing Loss in Children: A Review. JAMA 2020, 324, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Oxenham, A.J.; Micheyl, C.; Keebler, M.V.; Loper, A.; Santurette, S. Pitch perception beyond the traditional existence region of pitch. Proc. Natl. Acad. Sci. USA 2011, 108, 7629–7634. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Ito, K. Mechanisms of Bone-conducted Ultrasonic Perception Assessed by Measurements of Acoustic Fields in the Outer Ear Canal and Vibrations of the Tympanic Membrane. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 5962–5965. [Google Scholar] [CrossRef]
- Von Békésy, G. Experiments in Hearing; McGraw-Hill: New York, NY, USA, 1960. [Google Scholar]
- Bellucci, R.; Schuneider, D. Some observations on ultrasonic perception in man. Ann. Otol. Rhinol. Laryngol. 1962, 71, 719–726. [Google Scholar] [CrossRef]
- Nishimura, T.; Okayasu, T.; Uratani, Y.; Fukuda, F.; Saito, O.; Hosoi, H. Peripheral perception mechanism of ultrasonic hearing. Hear. Res. 2011, 277, 176–183. [Google Scholar] [CrossRef]
- Okayasu, T.; Nishimura, T.; Yamashita, A.; Saito, O.; Fukuda, F.; Yanai, S.; Hosoi, H. Human ultrasonic hearing is induced by a direct ultrasonic stimulation of the cochlea. Neurosci. Lett. 2013, 539, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Borse, V.A.; Aameri, R.F.H.; Sheehan, K.; Sheth, S.; Kaur, T.; Mukherjea, D.; Tupal, S.; Lowy, M.; Ghosh, S.; Dhukhwa, A.; et al. Epigallocatechin-3-gallate, a prototypic chemopreventative agent for protection against cisplatin-based ototoxicity. Cell Death Dis. 2017, 8, e2921. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Hirano, T.; Nishimura, T.; Nakagawa, S.; Watanabe, Y.; Hosoi, H.; Imaizumi, S.; Tonoike, M. Cerebral neuromagnetic responses evoked by two-channel bone-conducted ultrasound stimuli. In Proceedings of the 12th International Conference on Biomagnetism; Helsinki University of Technology: Espoo, Finland, 2001; pp. 121–124. [Google Scholar]
- Nishimura, T.; Sakaguchi, T.; Nakagawa, S.; Hosoi, H.; Watanabe, Y.; Tonoike, M.; Imaizumi, S. Dynamic range for bone conduction ultrasound. In Proceedings of the 12th International Conference on Biomagnetism; Helsinki University of Technology: Espoo, Finland, 2001; pp. 125–128. [Google Scholar]
- Nishimura, T.; Nakagawa, S.; Sakaguchi, T.; Hosoi, H.; Tonoike, M. Effect of stimulus duration for bone-conducted ultrasound on N1m in man. Neurosci. Lett. 2002, 327, 119–122. [Google Scholar] [CrossRef]
- Yamashita, A.; Nishimura, T.; Nakagawa, S.; Sakaguchi, T.; Hosoi, H. Assessment of ability to discriminate frequency of bone-conducted ultrasound by mismatch fields. Neurosci. Lett. 2008, 438, 260–262. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakagawa, S.; Yamashita, A.; Sakaguchi, T.; Hosoi, H. N1m amplitude growth function for bone-conducted ultrasound. Acta Otolaryngol. Suppl. 2009, 562, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, T.; Nishimura, T.; Uratani, Y.; Yamashita, A.; Nakagawa, S.; Yamanaka, T.; Hosoi, H.; Kitahara, T. Temporal window of integration estimated by omission in bone-conducted ultrasound. Neurosci. Lett. 2019, 696, 1–6. [Google Scholar] [CrossRef]
- Dhanasingh, A.; Jolly, C. An Overview of Cochlear Implant Electrode Array Designs. Hear. Res. 2017, 356, 93–103. [Google Scholar] [CrossRef] [PubMed]
- McRackan, T.R.; Bauschard, M.; Hatch, J.L.; Franko-Tobin, E.; Droghini, H.R.; Nguyen, S.A.; Dubno, J.R. Meta-Analysis of Quality-of-Life Improvement after Cochlear Implantation and Associations with Speech Recognition Abilities. Laryngoscope 2018, 128, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.A.; Noble, J.H.; Dawant, B.M.; Dwyer, R.T.; Labadie, R.F.; Gifford, R.H. Speech recognition as a function of the number of channels in perimodiolar electrode recipients. J. Acoust. Soc. Am. 2019, 145, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.A.; Prendergast, G.; Canavan, S. Measuring access to high-modulation-rate envelope speech cues in clinically fitted auditory prostheses. J. Acoust. Soc. Am. 2020, 147, 1284. [Google Scholar] [CrossRef]
- Warnecke, M.; Peng, Z.E.; Litovsky, R.Y. The impact of temporal fine structure and signal envelope on auditory motion perception. PLoS ONE. 2020, 15, e0238125. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Nakagawa, S.; Fujimoto, K.; Tonoike, M. Intelligibility of bone-conducted ultrasonic speech. Hear. Res. 2005, 208, 107–113. [Google Scholar] [CrossRef]
- Yamashita, A.; Nishimura, T.; Nagatani, Y.; Okayasu, T.; Koizumi, T.; Sakaguchi, T.; Hosoi, H. Comparison between bone-conducted ultrasound and audible sound in speech recognition. Acta. Otolaryngol. Suppl. 2009, 562, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Nishimura, T.; Nagatani, Y.; Sakaguchi, T.; Okayasu, T.; Yanai, S.; Hosoi, H. The effect of visual information in speech signals by bone-conducted ultrasound. NeuroReport 2009, 21, 119–122. [Google Scholar] [CrossRef]
- Dobie, R.A.; Wiederhold, M.L. Ultrasonic hearing. Science 1992, 255, 1584–1585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujimoto, K.; Nakagawa, S.; Tonoike, M. Nonlinear explanation for bone-conducted ultrasonic hearing. Hear. Res. 2005, 204, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kagomiya, T.; Nakagawa, S. An evaluation of bone-conducted ultrasonic hearing-aid regarding transmission of Japanese prosodic phonemes. In Proceedings of 20th International Congress on Acoustics; ICA: Sydney, Australia, 2010; pp. 23–27. [Google Scholar]
- Okayasu, T.; Nishimura, T.; Nakagawa, S.; Yamashita, A.; Nagatani, Y.; Uratani, Y.; Yamanaka, T.; Hosoi, H. Evaluation of prosodic and segmental change in speech-modulated bone-conducted ultrasound by mismatch fields. Neurosci. Lett. 2014, 559, 117–121. [Google Scholar] [CrossRef]
- Okayasu, T.; Nishimura, T.; Yamashita, A.; Nakagawa, S.; Nagatani, Y.; Yanai, S.; Uratani, Y.; Hosoi, H. Duration-dependent growth of N1m for speech-modulated bone-conducted ultrasound. Neurosci. Lett. 2011, 495, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Okayasu, T.; Saito, O.; Shimokura, R.; Yamashita, A.; Yamanaka, T.; Hosoi, H.; Kitahara, T. An examination of the effects of broadband air-conduction masker on the speech intelligibility of speech-modulated bone-conduction ultrasound. Hear. Res. 2014, 317, 41–49. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Edmondson-Jones, M.; Somerset, S.; Hall, D. A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 2016, 337, 70–79. [Google Scholar] [CrossRef]
- Szibor, A.; Mäkitie, A.; Aarnisalo, A.A. Tinnitus and suicide: An unresolved relation. Audiol. Res. 2019, 9, 222. [Google Scholar] [CrossRef]
- Ziai, K.; Moshtaghi, O.; Mahboubi, H.; Djalilian, H.R. Tinnitus patients suffering from anxiety and depression: A review. Int. Tinnitus J. 2017, 21, 68–73. [Google Scholar] [CrossRef]
- Tinnitus Retraining Therapy Trial Research Group; Scherer, R.W.; Formby, C. Effect of Tinnitus Retraining Therapy vs Standard of Care on Tinnitus-Related Quality of Life: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Sato, H.; Takahashi, M.; Wada, T.; Naito, Y.; Kawase, T.; Murakami, S.; Hara, A.; Kanzaki, S. Clinical practice guidelines for diagnosis and treatment of chronic tinnitus in Japan. Auris Nasus Larynx 2020, 47, 1–6. [Google Scholar] [CrossRef]
- Nagaraj, M.K.; Prabhu, P. Internet/smartphone-based applications for the treatment of tinnitus: A systematic review. Eur. Arch. Otorhinolaryngol. 2020, 277, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.E.; Moffat, G.; Bosnyak, D.J. Residual inhibition functions in relation to tinnitus spectra auditory threshold shift. Acta Otolaryngol. Suppl. 2006, 556, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.A.; Lenhardt, M.L.; Shulman, A. Tinnitus improvement with ultra high frequency vibration therapy. Int. Tinnitus J. 2005, 11, 14–22. [Google Scholar]
- Goldstein, B.A.; Shulman, A.; Lenhardt, M.L. Ultra-high frequency ultrasonic external acoustic stimulation for tinnitus relief: A method for patient selection. Int. Tinnitus J. 2005, 11, 111–114. [Google Scholar] [PubMed]
- Koizumi, T.; Nishimura, T.; Yamashita, A.; Yamanaka, T.; Imamura, T.; Hosoi, H. Residual inhibition of tinnitus induced by 30-kHz bone-conducted ultrasound. Hear. Res. 2014, 310, 48–53. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, T.; Okayasu, T.; Yamashita, A.; Hosoi, H.; Kitahara, T. Perception Mechanism of Bone-Conducted Ultrasound and Its Clinical Use. Audiol. Res. 2021, 11, 244-253. https://doi.org/10.3390/audiolres11020022
Nishimura T, Okayasu T, Yamashita A, Hosoi H, Kitahara T. Perception Mechanism of Bone-Conducted Ultrasound and Its Clinical Use. Audiology Research. 2021; 11(2):244-253. https://doi.org/10.3390/audiolres11020022
Chicago/Turabian StyleNishimura, Tadashi, Tadao Okayasu, Akinori Yamashita, Hiroshi Hosoi, and Tadashi Kitahara. 2021. "Perception Mechanism of Bone-Conducted Ultrasound and Its Clinical Use" Audiology Research 11, no. 2: 244-253. https://doi.org/10.3390/audiolres11020022
APA StyleNishimura, T., Okayasu, T., Yamashita, A., Hosoi, H., & Kitahara, T. (2021). Perception Mechanism of Bone-Conducted Ultrasound and Its Clinical Use. Audiology Research, 11(2), 244-253. https://doi.org/10.3390/audiolres11020022






