Anterior Column Reconstruction of the Thoracolumbar Spine with a Modular Carbon-PEEK Vertebral Body Replacement Device: Single-Center Retrospective Case Series of 28 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
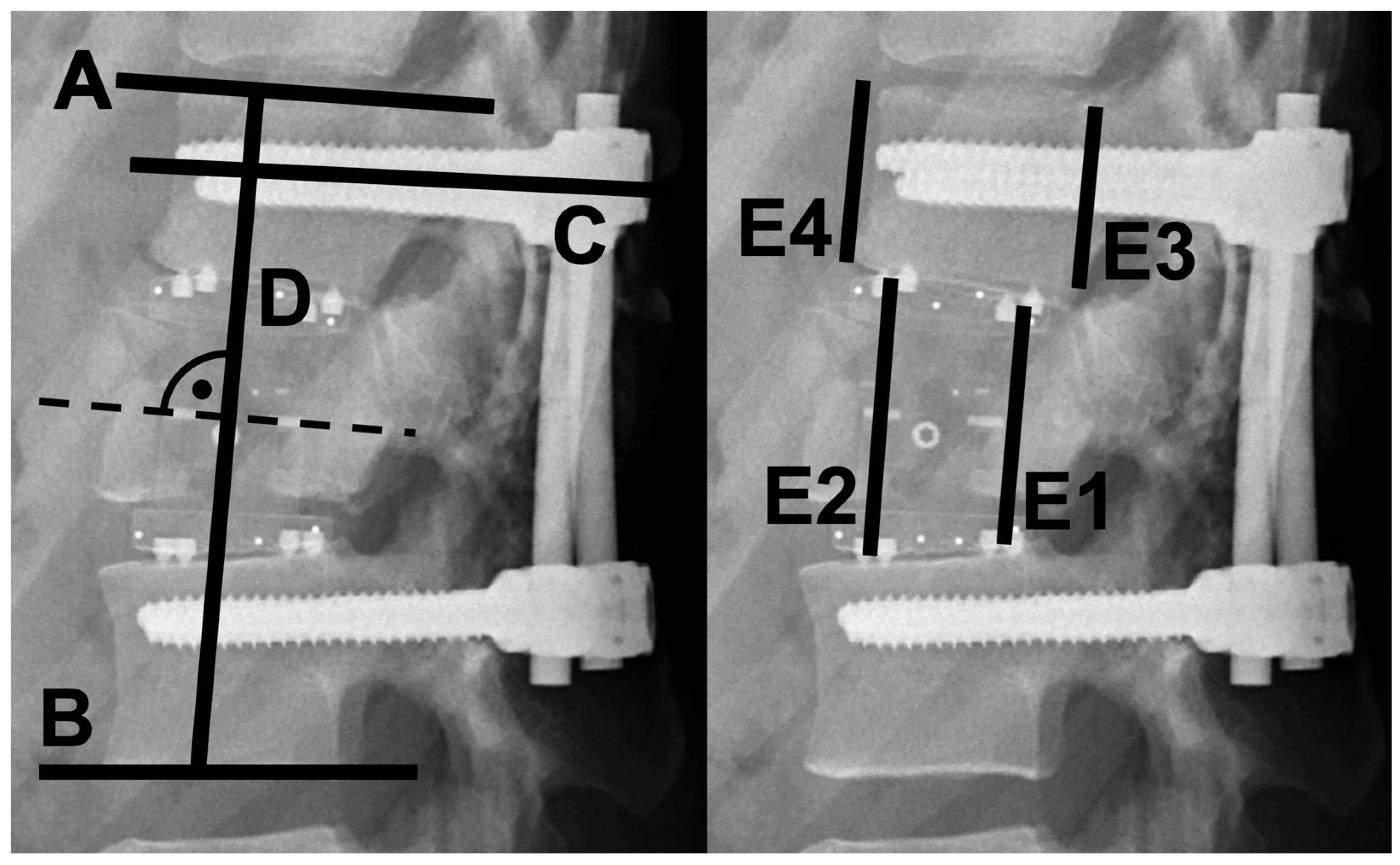
2.2. Radiographic Evaluation
2.3. Clinical Outcomes
2.4. Surgical Technique
2.5. Statistical Analyses
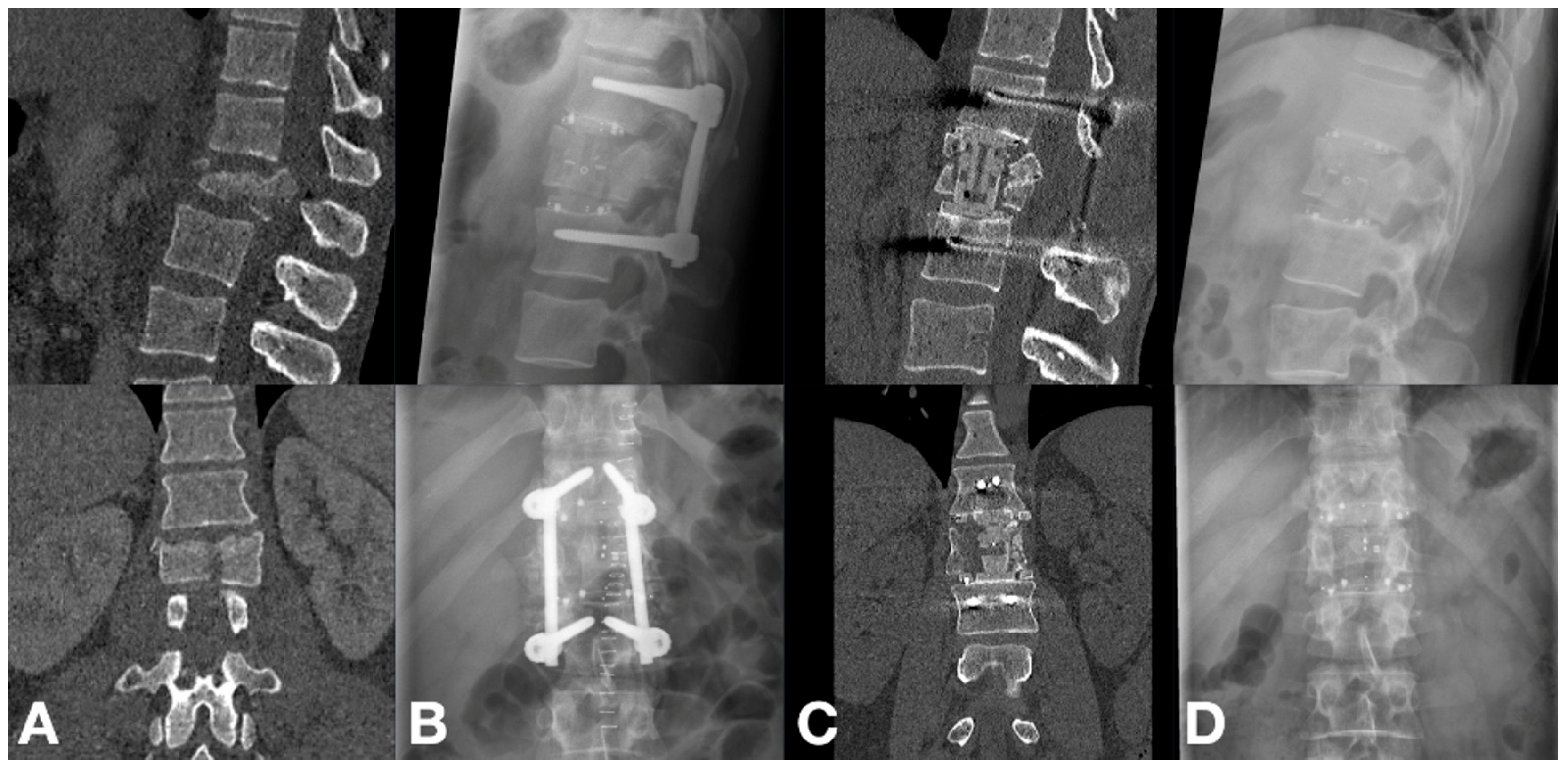
3. Results
3.1. Cohort Characteristics
3.2. Radiographic Outcomes
3.3. Clinical Outcomes
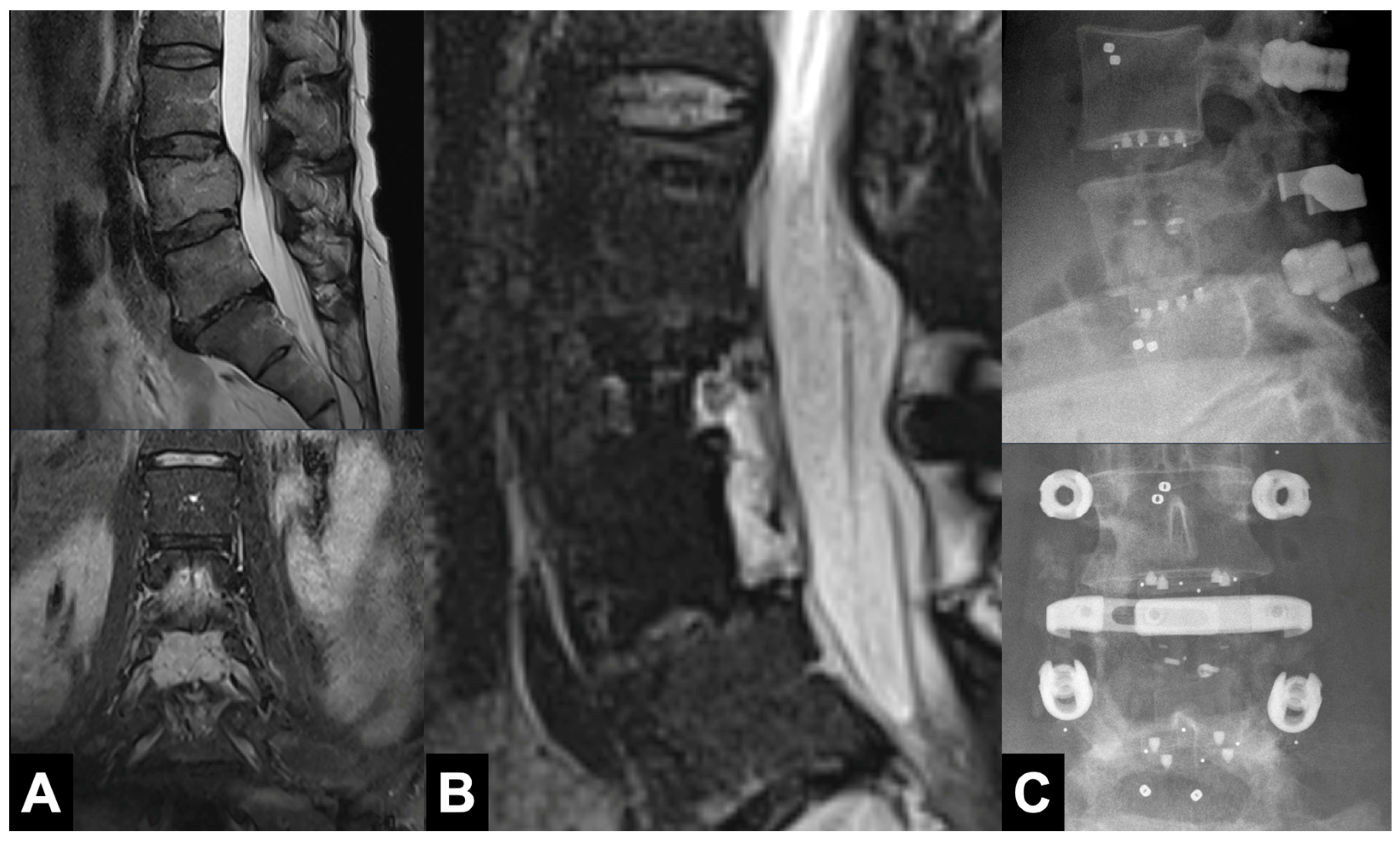
3.4. Complications
4. Discussion
4.1. Comparison with Existing Literature
4.2. Technical Aspects
4.3. Osseointegration and Fusion Rates
4.4. Rationale for Use and Economic Considerations
4.5. Implantation Safety and Complications
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | Anterior Column Reconstruction |
| AO Spine | Arbeitsgemeinschaft für Osteosynthesefragen Spine |
| APC | Article Processing Charge |
| ASIA | American Spinal Injury Association impairment scale |
| BKA | Bi-segmental Kyphotic Angle |
| CFR-PEEK | Carbon-Fiber-Reinforced Polyether-Ether-Ketone |
| CT | Computed Tomography |
| IQR | Interquartile Range |
| KEK | Kantonale Ethikkommission (Cantonal Ethics Commission, Bern) |
| KPS | Karnofsky Performance Status |
| MRI | Magnetic Resonance Imaging |
| PEEK | Polyether-Ether-Ketone |
| PROST | Patient-Reported Outcome Spine Trauma (AO Spine questionnaire) |
| SD | Standard Deviation |
| SINS | Spinal Instability Neoplastic Score |
| STIR | Short Tau Inversion Recovery |
| VBR | Vertebral-Body Replacement |
References
- Eleraky, M.; Papanastassiou, I.; Tran, N.D.; Dakwar, E.; Vrionis, F.D. Comparison of polymethylmethacrylate versus expandable cage in anterior vertebral column reconstruction after posterior extracavitary corpectomy in lumbar and thoraco-lumbar metastatic spine tumors. Eur. Spine J. 2011, 20, 1363–1370. [Google Scholar] [CrossRef]
- Schnake, K.J.; Stavridis, S.I.; Kandziora, F. Five-year clinical and radiological results of combined anteroposterior stabilization of thoracolumbar fractures. J. Neurosurg. Spine 2014, 20, 497–504. [Google Scholar] [CrossRef]
- Sacks, S. Anterior Interbody Fusion of the Lumbar Spine. J. Bone Jt. Surg. Br. Vol. 1965, 47-B, 211–223. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U.; Hecht, A.C.; DeLaney, T.F.; Chapman, P.A.; Hornicek, F.J.; Pedlow, F.X. Anterior Spinal Arthrodesis With Structural Cortical Allografts and Instrumentation for Spine Tumor Surgery. Spine 2004, 29, 1150–1158. [Google Scholar] [CrossRef]
- Grob, D.; Daehn, S.; Mannion, A.F. Titanium mesh cages (TMC) in spine surgery. Eur. Spine J. 2005, 14, 211–221. [Google Scholar] [CrossRef]
- Kumar, N.; Ramakrishnan, S.A.; Lopez, K.G.; Madhu, S.; Ramos, M.R.D.; Fuh, J.Y.H.; Hallinan, J.; Nolan, C.P.; Benneker, L.M.; Vellayappan, B.A. Can Polyether Ether Ketone Dethrone Titanium as the Choice Implant Material for Metastatic Spine Tumor Surgery? World Neurosurg. 2021, 148, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, N.; Yolcu, Y.U.; Alan, N.; Agarwal, N.; Hamilton, D.K.; Ozpinar, A. Evolution of polyetheretherketone (PEEK) and titanium interbody devices for spinal procedures: A comprehensive review of the literature. Eur. Spine J. 2022, 31, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.T.; Richards, A.E.; Curley, K.L.; Patel, N.P.; Ashman, J.B.; Vora, S.A.; Kalani, M.A. Carbon fiber–reinforced PEEK instrumentation in the spinal oncology population: A retrospective series demonstrating technique, feasibility, and clinical outcomes. Neurosurg. Focus 2021, 50, E13. [Google Scholar] [CrossRef] [PubMed]
- Hubertus, V.; Wessels, L.; Früh, A.; Tkatschenko, D.; Nulis, I.; Bohner, G.; Prinz, V.; Onken, J.; Czabanka, M.; Vajkoczy, P.; et al. Navigation accuracy and assessability of carbon fiber-reinforced PEEK instrumentation with multimodal intraoperative imaging in spinal oncology. Sci. Rep. 2022, 12, 15816. [Google Scholar] [CrossRef]
- Nevelsky, A.; Borzov, E.; Daniel, S.; Bar-Deroma, R. Perturbation effects of the carbon fiber-PEEK screws on radiotherapy dose distribution. J. Appl. Clin. Med. Phys. 2017, 18, 62–68. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Bai, X.; Wang, Y.; Han, P.; Li, H. Titanium-coated polyetheretherketone cages vs. polyetheretherketone cages in lumbar interbody fusion: A systematic review and meta-analysis. Exp. Ther. Med. 2023, 25, 305. [Google Scholar] [CrossRef]
- Schwendner, M.; Ille, S.; Kirschke, J.S.; Bernhardt, D.; Combs, S.E.; Meyer, B.; Krieg, S.M. Clinical evaluation of vertebral body replacement of carbon fiber-reinforced polyetheretherketone in patients with tumor manifestation of the thoracic and lumbar spine. Acta Neurochir 2023, 165, 897–904. [Google Scholar] [CrossRef]
- Sadiqi, S.; Verlaan, J.-J.; Lehr, A.M.; Chapman, J.R.; Dvorak, M.F.; Kandziora, F.; Rajasekaran, S.; Schnake, K.J.; Vaccaro, A.R.; Oner, F.C. Measurement of kyphosis and vertebral body height loss in traumatic spine fractures: An international study. Eur. Spine J. 2017, 26, 1483–1491. [Google Scholar] [CrossRef]
- Aghayev, E.; Zullig, N.; Diel, P.; Dietrich, D.; Benneker, L.M. Development and validation of a quantitative method to assess pedicle screw loosening in posterior spine instrumentation on plain radiographs. Eur. Spine J. 2014, 23, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Deml, M.C.; Mazuret Sepulveda, C.; Albers, C.; Hoppe, S.; Bigdon, S.; Häckel, S.; Milavec, H.; Benneker, L.M. Anterior column reconstruction of the thoracolumbar spine with a new modular PEEK vertebral body replacement device: Retrospective clinical and radiologic cohort analysis of 48 cases with 1.7-years follow-up. Eur. Spine J. 2020, 29, 3194–3202. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, K.H.; Lenke, L.G.; McEnery, K.W.; Baldus, C.; Blanke, K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine 1995, 20, 1410–1418. [Google Scholar] [CrossRef]
- Eck, K.R.; Lenke, L.G.; Bridwell, K.H.; Gilula, L.A.; Lashgari, C.J.; Riew, K.D. Radiographic assessment of anterior titanium mesh cages. Clin. Spine Surg. 2000, 13, 501–509. [Google Scholar] [CrossRef]
- Guggenberger, R.; Winklhofer, S.; Osterhoff, G.; Wanner, G.; Fortunati, M.; Andreisek, G.; Alkadhi, H.; Stolzmann, P. Metallic artefact reduction with monoenergetic dual-energy CT: Systematic ex vivo evaluation of posterior spinal fusion implants from various vendors and different spine levels. Eur. Radiol. 2012, 22, 2357–2364. [Google Scholar] [CrossRef]
- Bamberg, F.; Dierks, A.; Nikolaou, K.; Reiser, M.F.; Becker, C.R.; Johnson, T.R. Metal artifact reduction by dual energy computed tomography using monoenergetic extrapolation. Eur. Radiol. 2011, 21, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Odom, G.L.; Finney, W.; Woodhall, B. Cervical disk lesions. J. Am. Med. Assoc. 1958, 166, 23–28. [Google Scholar] [CrossRef]
- Shi, L.; Su, Y.; Yan, T.; Wang, H.; Wang, K.; Liu, L. Early microsurgery on thoracolumbar spinal extradural arachnoid cysts: Analysis of a series of 41 patients. J. Clin. Neurosci. 2021, 94, 257–265. [Google Scholar] [CrossRef]
- Häckel, S.; Oswald, K.A.C.; Koller, L.; Benneker, L.M.; Benneker, L.A.; Sadiqi, S.; Oner, F.C.; Deml, M.C. Reliability and Validity of the German Version of the AO Spine Patient Reported Outcome Spine Trauma Questionnaire. Glob. Spine J. 2023, 14, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Péus, D.; Newcomb, N.; Hofer, S. Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med. Inform. Decis. Mak. 2013, 13, 72. [Google Scholar] [CrossRef]
- Lange, U.; Edeling, S.; Knop, C.; Bastian, L.; Oeser, M.; Krettek, C.; Blauth, M. Anterior vertebral body replacement with a titanium implant of adjustable height: A prospective clinical study. Eur. Spine J. 2007, 16, 161–172. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Lu, X.; Yang, L.; Yang, H.; Yuan, W.; Chen, D. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: A prospective, randomized, control study with over 7-year follow-up. Eur. Spine J. 2013, 22, 1539–1546. [Google Scholar] [CrossRef]
- Lowe, T.G.; Hashim, S.; Wilson, L.A.; O’Brien, M.F.; Smith, D.A.B.; Diekmann, M.J.; Trommeter, J. A Biomechanical Study of Regional Endplate Strength and Cage Morphology as It Relates to Structural Interbody Support. Spine 2004, 29, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Torstrick, F.B.; Lin, A.S.P.; Safranski, D.L.; Potter, D.; Sulchek, T.; Lee, C.S.D.; Gall, K.; Guldberg, R.E. Effects of Surface Topography and Chemistry on Polyether-Ether-Ketone (PEEK) and Titanium Osseointegration. Spine 2020, 45, E417–E424. [Google Scholar] [CrossRef] [PubMed]
- Brandao, R.C.S.; Martins, W.d.S.; Arantes, A.; Gusmão, S.S.; Perrin, G.; Barrey, C. Titanium versus polyetheretherketone implants for vertebral body replacement in the treatment of 77 thoracolumbar spinal fractures. Surg. Neurol. Int. 2017, 8, 191. [Google Scholar] [CrossRef]
- Seaman, S.; Kerezoudis, P.; Bydon, M.; Torner, J.C.; Hitchon, P.W. Titanium vs. polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J. Clin. Neurosci. 2017, 44, 23–29. [Google Scholar] [CrossRef]
- Kienle, A.; Graf, N.; Wilke, H.-J. Does impaction of titanium-coated interbody fusion cages into the disc space cause wear debris or delamination? Spine J. 2016, 16, 235–242. [Google Scholar] [CrossRef]
- Torstrick, F.B.; Klosterhoff, B.S.; Westerlund, L.E.; Foley, K.T.; Gochuico, J.; Lee, C.S.; Gall, K.; Safranski, D.L. Impaction durability of porous polyether-ether-ketone (PEEK) and titanium-coated PEEK interbody fusion devices. Spine J. 2018, 18, 857–865. [Google Scholar] [CrossRef] [PubMed]


| Variable | Trauma (n = 15) | Osteoporotic (n = 7) | Tumors (n = 6) | Total (n = 28) |
|---|---|---|---|---|
| Age, mean ± SD a (years) | 54.5 ± 18.4 | 77.7 ± 9.8 | 62.5 ± 14.0 | 60.5 ± 18.6 |
| Female sex, n (%) | 5 (33.3%) | 3 (42.9%) | 2 (33.3%) | 10 (35.7%) |
| Fracture Classifications | ||||
| AO Spine Classification | ||||
| Type A | 9 | — | — | 9 |
| Type B | 4 | — | — | 4 |
| Type C | 2 | — | — | 2 |
| AO Spine OF b Classification | ||||
| OF 3 | — | 1 | — | 1 |
| OF 4 | — | 6 | — | 6 |
| SINS c Classification | ||||
| Score 0–6 | — | — | 1 | 1 |
| Score 7–12 | — | — | 3 | 3 |
| Score 13–18 | — | — | 2 | 2 |
| Initial Neurological Impairment (ASIA d Grade) | ||||
| ASIA A | 1 | 0 | 0 | 1 |
| ASIA B | 0 | 0 | 0 | 0 |
| ASIA C | 3 | 0 | 2 | 5 |
| ASIA D | 0 | 0 | 2 | 2 |
| ASIA E | 11 | 7 | 2 | 20 |
| Number of Spinal Levels Instrumented | ||||
| Less than 3 levels | 0 | 1 | 0 | 1 |
| 3 levels | 8 | 3 | 2 | 13 |
| More than 3 levels | 7 | 3 | 4 | 14 |
| Staging of Surgeries | ||||
| 1-stage surgery | 2 | 5 | 4 | 11 |
| 2-stage surgery | 13 | 2 | 2 | 17 |
| Bisegmental Kyphotic Angle, Mean ± SD, in Degrees | |||||||
|---|---|---|---|---|---|---|---|
| Group | T1 a | T2 b | T3 c | p-Values d | |||
| Whole | −11.6 ± 20.4 | 4.9 ± 20.5 | 2.9 ± 18.0 | T1 vs. T2: 0.006 * T2 vs. T3: 0.568 T1 vs. T3: 0.008 * | |||
| Trauma | −10.8 ± 16.7 | −2.2 ± 16.7 | −1.1 ± 18.7 | T1 vs. T2: 0.234 T2 vs. T3: 0.837 T1 vs. T3: 0.178 | |||
| Osteoporotic | −16.3 ± 23.8 | 5.5 ± 13.2 | 2.8 ± 13.6 | T1 vs. T2: 0.009 * T2 vs. T3: 0.072 T1 vs. T3: 0.021 * | |||
| Tumor | −2.5 ± 41.7 | 45.0 ± 14.4 | 27.0 ± 4.2 | T1 vs. T2: 0.248 T2 vs. T3: 0.236 T1 vs. T3: 0.466 | |||
| Sagittal tilt, mean ± sd, in degrees | |||||||
| Group | T1 | T3 | p-values d | ||||
| Whole | 88.9 ± 9.7 | 88.1 ± 10.4 | T1 vs. T3: 0.39 | ||||
| Epsilon angle, mean ± sd, in degrees | |||||||
| Group | T1 | T3 | p-values d | ||||
| Whole | 4.8 ± 4.0 | 5.0 ± 3.5 | T1 vs. T3: 0.80 | ||||
| Construct height, mean ± sd, in mm | |||||||
| Group | T1 | T3 | p-values d | ||||
| Whole | 106.2 ± 12.9 | 105.7 ± 13.2 | T1 vs. T3: 0.052 | ||||
| Cage height coefficient, median (IQR) | |||||||
| Group | T1 | T3 | p-values e | ||||
| Whole | 1.7 (0.36) | 1.6 (0.35) | T1 vs. T3: 0.140 | ||||
| Grade | Description of Fusion | n (%) |
|---|---|---|
| Bridwell classification (computed tomography) | Total n = 18 | |
| I | Definite (fused with remodeling and trabeculae) | 14 (78%) |
| II | Probable (graft intact, not fully remodeled and incorporated through; no lucencies) | 3 (17%) |
| III | Probably no (graft intact, but a definite lucency at the top or bottom of the graft) | 1 (5%) |
| IV | No (definitely not fused with resorption of bone graft and with collapse) | 0 |
| V | Could not be assessed | 0 |
| Bridwell Classification (plain radiograph) | Total n = 28 | |
| I | Definite (fused with remodeling and trabeculae) | 18 (64%) |
| II | Probable (graft intact, not fully remodeled and incorporated through; no lucencies) | 8 (29%) |
| III | Probably no (graft intact, but a definite lucency at the top or bottom of the graft) | 2 (7%) |
| IV | No (definitely not fused with resorption of bone graft and with collapse) | 0 |
| V | Could not be assessed | 0 |
| Metric | Carbon Cage (Mean ± SD a) | Titanium Screw (Mean ± SD) | p-Value |
|---|---|---|---|
| Artifact Width (mm) | 1.4 ± 0.5 | 2.6 ± 0.8 | 0.0004 |
| Artifact HU b | −46.8 ± 39.7 | −360.0 ± 104.1 | <0.0001 |
| Artifact SD | 57.2 ± 49.8 | 127.7 ± 47.7 | 0.0010 |
| Absolute Difference (HU) | 109.4 ± 56.6 | 409.5 ± 107.0 | <0.0001 |
| Relative Density (%) | −87.6 ± 95.1 | −946.2 ± 581.3 | 0.0001 |
| Implant HU | 352.7 ± 23.4 | 3068.6 ± 3.9 | <0.0001 |
| Outcome Measure | Preoperative | 2 Months | 6 Months | Last |
|---|---|---|---|---|
| A. Odom’s Criteria (Non-Tumor Patients, n = 22) | ||||
| Excellent, n (%) | — | 4 (18.2%) | 10 (45.5%) | 13 (59.1%) |
| Good, n (%) | — | 11 (50.0%) | 10 (45.5%) | 7 (31.8%) |
| Fair, n (%) | — | 6 (27.3%) | 2 (9.1%) | 1 (4.5%) |
| Poor, n (%) | — | 1 (4.5%) | 0 (0.0%) | 1 (4.5%) |
| B. AOSpine PROST a Score (Trauma Patients, n = 15) | ||||
| Mean ± SD | — | — | — | 56.9 ± 21.3 |
| C. Karnofsky Performance Status (Tumor Patients, n = 6) | ||||
| Median (IQR) | 60.0 (15.0) | — | — | 80.0 (15.0) |
| D. ASIA b Impairment Scale (Patients with Initial Deficits, n = 8) | ||||
| Patient 1 (Trauma) | A | A | A | A |
| Patient 2 (Trauma) | C | D | D | D |
| Patient 3 (Trauma) | C | D | D | D |
| Patient 4 (Trauma) | C | D | D | E |
| Patient 5 (Tumor) | C | D | D | D |
| Patient 6 (Tumor) | C | E | E | E |
| Patient 7 (Tumor) | D | E | E | E |
| Patient 8 (Tumor) | D | E | E | E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaible, S.F.; Aregger, F.C.; Albers, C.E.; Benneker, L.M.; Deml, M.C. Anterior Column Reconstruction of the Thoracolumbar Spine with a Modular Carbon-PEEK Vertebral Body Replacement Device: Single-Center Retrospective Case Series of 28 Patients. Surg. Tech. Dev. 2025, 14, 35. https://doi.org/10.3390/std14040035
Schaible SF, Aregger FC, Albers CE, Benneker LM, Deml MC. Anterior Column Reconstruction of the Thoracolumbar Spine with a Modular Carbon-PEEK Vertebral Body Replacement Device: Single-Center Retrospective Case Series of 28 Patients. Surgical Techniques Development. 2025; 14(4):35. https://doi.org/10.3390/std14040035
Chicago/Turabian StyleSchaible, Samuel F., Fabian C. Aregger, Christoph E. Albers, Lorin M. Benneker, and Moritz C. Deml. 2025. "Anterior Column Reconstruction of the Thoracolumbar Spine with a Modular Carbon-PEEK Vertebral Body Replacement Device: Single-Center Retrospective Case Series of 28 Patients" Surgical Techniques Development 14, no. 4: 35. https://doi.org/10.3390/std14040035
APA StyleSchaible, S. F., Aregger, F. C., Albers, C. E., Benneker, L. M., & Deml, M. C. (2025). Anterior Column Reconstruction of the Thoracolumbar Spine with a Modular Carbon-PEEK Vertebral Body Replacement Device: Single-Center Retrospective Case Series of 28 Patients. Surgical Techniques Development, 14(4), 35. https://doi.org/10.3390/std14040035





