2. Surgical Technique
Under general anesthesia and in lateral decubitus with lumbar hyperextension, the required anatomical landmarks for the procedure are identified and marked (
Figure 1A,B). The incision should be slightly cranial and anterior to the classic lumbotomy approach since precise access over the ureteropelvic junction simplifies the procedure. A 12–14 mm transverse incision is made, and access to the retroperitoneum is achieved by blunt dissection (muscle-sparing) until Gerota’s fascia is reached. It is essential not to be directed anteriorly during dissection to avoid inadvertently opening the parietal peritoneum. Once the retroperitoneum has been accessed, a blunt digital dissection of the perirenal space is performed, and an Alexis
® XXS wound protector–retractor (Applied Medical, Rancho Santa Margarita, CA, USA) is placed. Subsequently, a gloveport with three 5 mm working channels is placed (
Figure 1C). A size 5.5 glove is used to prepare the gloveport. We make longitudinal cuts at the tip of each finger, usually on the first, third, and fourth fingers (
Figure 2A). Then, we introduce the trocars up to the central area of the glove (
Figure 2B) and secure the trocars to the glove with a sterile adhesive strip to prevent CO
2 leakage (
Figure 2C). Introducing the trocars up to the glove’s central area facilitates instrument insertion and expedites the surgery. To create a tight and firm connection between the Alexis
® and the glove, the Alexis
® system is placed in the surgical field without completing the outer ring’s last 2–3 turns. The glove is then placed wrapped around the Alexis® outer ring (
Figure 2D), and the last 2–3 outer ring turns are completed. This allows the glove to be partially wrapped around the Alexis
® (
Figure 2E) and prevents accidental detachment after CO
2 insufflation.
CO
2 insufflation should ideally be set at no more than 8–9 mmHg pressure and 1–1.5 L per minute flow. These parameters allow a pneumodissection of the perirenal space and an adequate working cavity (
Figure 3A). The lower renal pole should be identified (
Figure 3B) and a blunt dissection should be performed until the ureter is identified (
Figure 3B,C). This dissection is performed with Endo-peanuts
® (laparoscopic swabs) (Medtronic, Minneapolis, MN, USA), but any 5 mm laparoscopic instrument can be used. It should be considered that excessive dissection of the kidney may lead to excessive mobility, hindering the procedure. The gloveport system provides excellent camera mobility (in our case, we use a 5 mm and 30-degree optic), and the retroperitoneoscopic part of the procedure can be performed from different angles according to the surgeon’s comfort and preferences. Likewise, two working channels allow bimanual blunt dissection (
Figure 3C) and parallel use of intelligent coagulation instruments such as LigaSure™ (Medtronic, Minneapolis, MN, USA) (
Figure 3D). Ureter dissection should continue until adequate mobilization is confirmed (
Figure 3E). After that, the ureter is secured with a silicone loop (vessel loop), allowing for its externalization (
Figure 3F,G). The glove is disassembled without removing the Alexis
®, and the Alexis
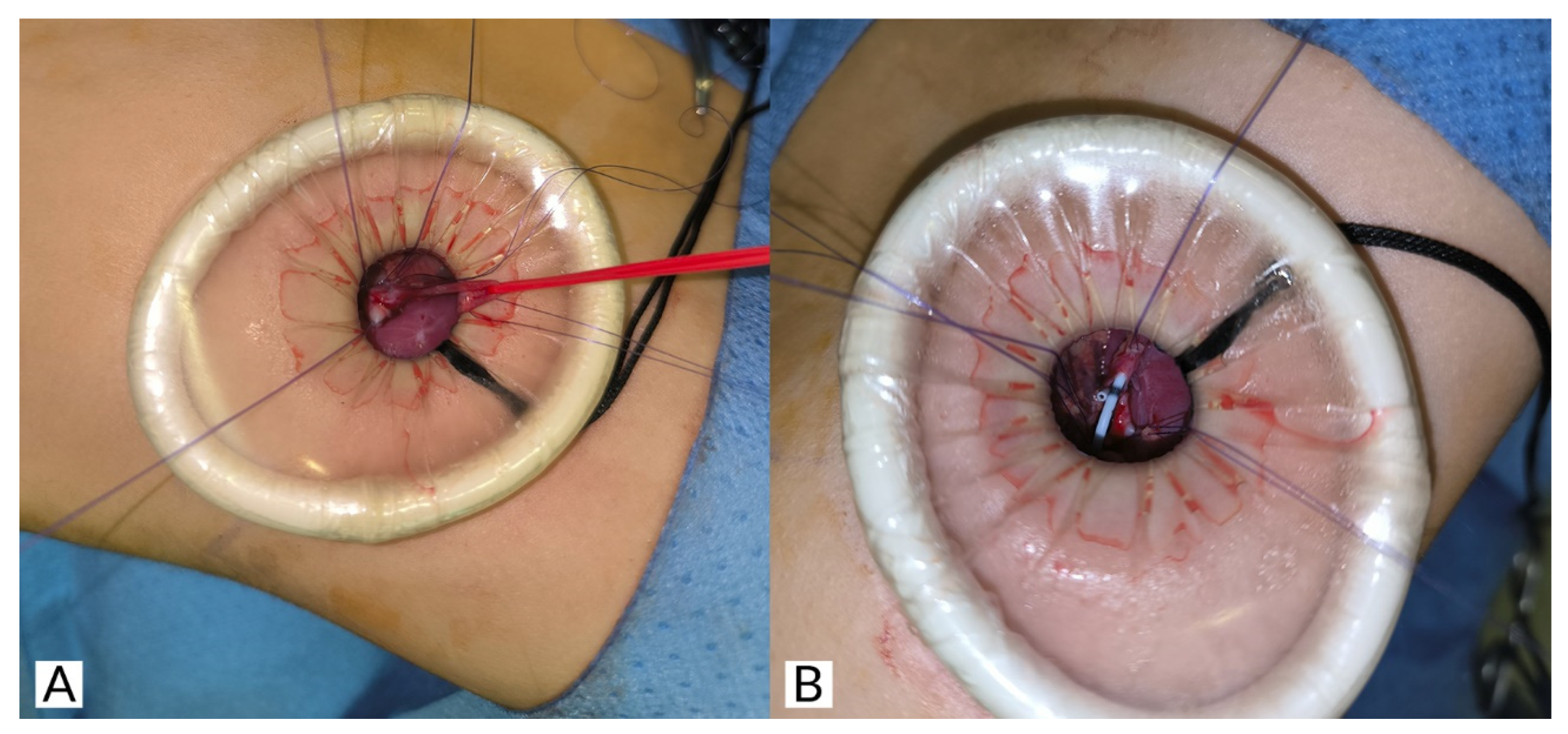
® is retightened with 2–3 turns. Then, lateral sutures are applied to the ureter and pelvis. These sutures are applied externally and under direct vision, which is much simpler than if performed laparoscopically/retroperitoneoscopically. After this, a standard dismembered pyeloplasty is performed (
Figure 4A,B). We use a double-J stent as a transanastomotic tutor, making its placement through this surgical access easy (
Figure 4B). After the anastomosis is completed, the surgical cavity is inspected (
Figure 3H), a surgical drain is placed, and the wound is closed in a standard fashion (
Figure 1D).
3. Comments
The retroperitoneal approach for UPJO surgical correction constitutes a complex technique for multiple reasons: (1) The spatial arrangement of renal anatomy is difficult to interpret from this perspective and requires specific training. (2) The retroperitoneal cavity is smaller than the peritoneal one and, therefore, the laparoscopic triangulation and the endocavitary part of the procedure are more complex. (3) This surgical field includes structures such as the renal vessels and the aorta that pose a vital risk in case of damage. All of these challenges are amplified when using the present gloveport technique since (1) the triangulation capacity is diminished compared with conventional laparoscopy and (2) the long trajectory of the gloveport fingers increases the risk of kinking during insertion, potentially leading to glove perforation. Nevertheless, the present technique (gloveport-assisted retroperitoneal pyeloplasty or GARP) also has notable advantages: it protects the wound’s surgical edges, enables more precise and faster bimanual endocavitary work, and minimizes muscle dissection and scarring, potentially leading to superior functional and aesthetic outcomes compared to other techniques. However, this last aspect is beyond the scope of this study and has not been rigorously evaluated.
The learning curve for the GARP technique is considered complex. The main author of this manuscript previously developed extensive experience in multiple single-port pediatric surgical procedures, which facilitated the acquisition of this specific competence. Gaining proficiency in simpler and more common single-port procedures under appropriate mentoring, such as transumbilical video-assisted appendectomies (TULAA), is essential for the new generation of pediatric surgeons to master this technique.
Replacing the gloveport with a commercial gelport is an appealing alternative, as it eliminates the risk of glove perforation and facilitates instrument insertion. The trocar path in gelport systems is typically shorter and more rigid, without the fragile areas present in gloveport systems. On the other hand, the internal ring of the Alexis-type retraction system included in commercial gelports is usually larger. This may produce greater surgical wound distraction, enlarging its size. Moreover, the cost of commercial gelport systems is significantly higher than that of the gloveport system. Cost-effectiveness studies are required to assess this aspect rigorously.
It is important to emphasize the assistant’s role in this procedure. On the one hand, given the limited working space and triangulation capacity, camera movements must be minimal and precise, as it is easy to accidentally dislodge the optics from the cavity. On the other hand, the surgeon’s intermittent introduction of new instruments through the working channels requires the assistant’s intervention since it is easy to pierce the gloveport with the instruments inadvertently in the path between the trocar and the surgical wound (which constitutes the exposed piece of glove). If this occurs, the CO2-insufflated cavity is lost. This can be solved by placing an adhesive in the pierced area or replacing the gloveport. To prevent glove perforation, the assistant guides the instruments through the trocar with their hand from outside the glove, ensuring a smooth introduction into the cavity. The assistant should be an experienced surgeon during the initial learning curve of the procedure.
We have not suffered any intraoperative complications with this technique. However, the primary risk or potential complication of this procedure is damage to adjacent structures, particularly the renal hilum. This complication, common to all minimally invasive surgical techniques for UPJO, makes it potentially necessary to convert quickly to open surgery. This risk should be carefully considered during preoperative planning.
Although no comparative studies exist between video-assisted single-incision techniques for this pathology (OTAP, RoTAP, and the present novel variation), this represents a potential field of interest for future research. We hypothesize that this technique may have a lower conversion rate to open surgery in cases of technical difficulty, given the possibility of performing bimanual endocavitary work; however, this has not been demonstrated. Similarly, when comparing these techniques, medium- and long-term renal functional outcomes and complications (such as strictures) are also relevant parameters that need to be characterized.
Finally, in our case, the cost of the gloveport system is approximately 120 euros. Notably, the gloveport system does not require 10–12 mm trocars or trocars with an introducer, substantially reducing costs compared to conventional laparoscopy. Its assembly takes only two minutes with an experienced team, and the technique does not incur any additional costs compared to conventional laparoscopy.
The main strengths of this study are its comprehensive literature review and the detailed, reproducible description of the technique. As a major limitation, this work constitutes a technical note based on very limited unicentric casuistry. Additionally, we have not conducted any comparative analysis between GARP and other reference techniques for UPJO; therefore, these findings should be interpreted with caution.
In conclusion, GARP may be a safe and cost-effective surgical alternative to laparoscopic pyeloplasty, particularly in infants. The ideal candidates for this technique are infants and low-weight patients, for whom the laparoscopic/robotic approach presents a significant technical challenge, making this technique a fast and effective alternative. Our experience is preliminary, and further studies are required to refine this technique and validate its clinical utility in this pathology.