Three-Dimensional Computed Tomography-Assisted Complex Lung Segmentectomies for Challenging Oncological Cases
Abstract
1. Introduction
2. Case Presentation
2.1. Case 1
2.2. Case 2
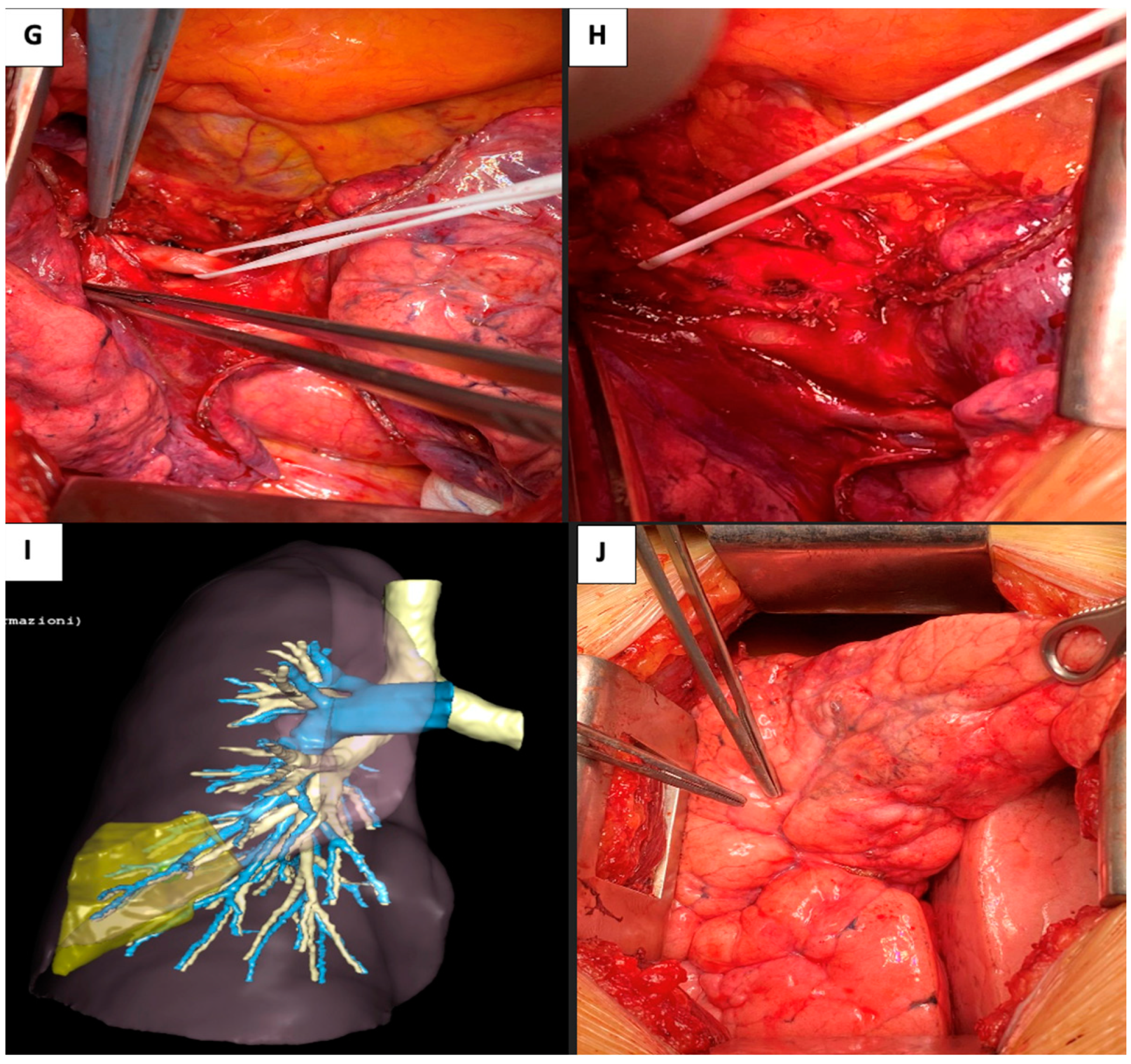
2.3. Case 3
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
- Nomori, H.; Mori, T.; Ikeda, K.; Yoshimoto, K.; Iyama, K.; Suzuki, M. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: A prospective study at a single institute. J. Thorac. Cardiovasc. Surg. 2012, 144, 87–93. [Google Scholar] [CrossRef]
- Oizumi, H.; Endoh, M.; Takeda, S.-I.; Suzuki, J.; Fukaya, K.; Sadahiro, M. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann. Thorac. Surg. 2010, 90, 1382–1383. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, K.; Han, X.; Zhao, J.; Wang, G.; Yuan, S.; He, B. Three-dimensional computed tomography angiography and bronchography combined with three-dimensional printing for thoracoscopic pulmonary segmentectomy in stage IA non-small cell lung cancer. J. Thorac. Dis. 2021, 13, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Iwano, S.; Yokoi, K.; Taniguchi, T.; Kawaguchi, K.; Fukui, T.; Naganawa, S. Planning of segmentectomy using three-dimensional computed tomography angiography with a virtual safety margin: Technique and initial experience. Lung Cancer 2013, 81, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Wang, J.; Yao, J.; Hang, F.; Lei, X.; Cao, Y. Three-dimensional image reconstruction with free open-source OsiriX software in video-assisted thoracoscopic lobectomy and segmentectomy. Int. J. Surg. 2017, 39, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Baste, J.M.; Soldea, V.; Lachkar, S.; Rinieri, P.; Sarsam, M.; Bottet, B.; Peillon, C. Development of a precision multimodal surgical navigation system for lung robotic segmentectomy. J. Thorac. Dis. 2018, 10 (Suppl. S10), S1195–S1204. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, J.; Peillon, C.; Dacher, J.-N.; Baste, J.-M. Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: A pilot study. J. Thorac. Dis. 2018, 10, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, Z.; Qin, Y.; Jiao, W. Progress in three-dimensional computed tomography reconstruction in anatomic pulmonary segmentectomy. Thorac. Cancer 2022, 13, 1881–1887. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, R.; Gherzi, L.; Ferrari, M.; Mattioni, G.; Alifano, M.; Pardolesi, A. Three-Dimensional Computed Tomography-Assisted Complex Lung Segmentectomies for Challenging Oncological Cases. Surg. Tech. Dev. 2024, 13, 269-277. https://doi.org/10.3390/std13030020
Orlandi R, Gherzi L, Ferrari M, Mattioni G, Alifano M, Pardolesi A. Three-Dimensional Computed Tomography-Assisted Complex Lung Segmentectomies for Challenging Oncological Cases. Surgical Techniques Development. 2024; 13(3):269-277. https://doi.org/10.3390/std13030020
Chicago/Turabian StyleOrlandi, Riccardo, Lorenzo Gherzi, Michele Ferrari, Giovanni Mattioni, Marco Alifano, and Alessandro Pardolesi. 2024. "Three-Dimensional Computed Tomography-Assisted Complex Lung Segmentectomies for Challenging Oncological Cases" Surgical Techniques Development 13, no. 3: 269-277. https://doi.org/10.3390/std13030020
APA StyleOrlandi, R., Gherzi, L., Ferrari, M., Mattioni, G., Alifano, M., & Pardolesi, A. (2024). Three-Dimensional Computed Tomography-Assisted Complex Lung Segmentectomies for Challenging Oncological Cases. Surgical Techniques Development, 13(3), 269-277. https://doi.org/10.3390/std13030020







