Abstract
The current reconstructive surgical procedures implemented after the resection of extended bone segments are associated with high complication rates and long-term treatments. By transplanting an autologous, vascularized and stabilized bone segment, these challenges can be managed. Thus, we propose a novel procedure to expand the currently available autologous bone grafts to the dimensions of the recipient bone using an implantable device. The objective of the present study was to characterize the feasibility of developing an implant prototype for fibula expansion in an in vitro model using a porcine fibula. A balloon catheter, as the part of the implant responsible for expansion, was proven to expand while being periodically filled with sodium chloride. Therefore, the expansion of the balloon catheter was analyzed in an experimental test setup with a 3D-printed porcine fibula with a closure film simulating callus formation to simulate the in vivo situation. Our experimental testing proved the successful expansion of the porcine fibula by the balloon catheter. Hence, the feasibility of the concept for subsequent animal testing was confirmed.
1. Introduction
The reconstruction of long bone defects after the resection of extended bone segments due to a tumor or infection represents a major challenge in reconstructive surgery. The currently available procedures are associated with high complication rates, as well as revision rates of up to 60% [1,2,3]. Segmental bone transportation with callus distraction of more than 4 cm requires stabilization with an intramedullary nail or an external fixator, even though this increases the risk of pin tract infection [4,5,6,7,8,9]. Moreover, with an average healing index of approximately 50 days per cm of newly formed bone [4,5,10,11,12], the duration of treatment for the reconstruction of extended bone segments can take months or years, and several surgical interventions are usually required. In particular, the treatment of bone defects of 10 cm or more is associated with high complication rates [1,2,3]. The development and use of bone transport nails improved the possibilities for long bone defect reconstruction. The advantage of bone transport nails is internal fixation and transport; their disadvantages are high costs, limited size of reconstruction of about 8 cm in length and the need for an additional external power station. Further complications have not been described due to the limited number of implantations [13].
Alternatively, long bone defects resulting from infections can be treated with vascularized free fibular transfer. In cases of metadiaphyseal tumors, after tumor resection, free fibula transfer combined with an allograft was described by Capanna et al. [14] Because the defect of the bone was bridged by the transplanted fibula and the additional allograft, weightbearing was allowed once there is evidence of union of the transplant, usually after 2–3 months. With this method, the reconstruction of the defect was achieved without mechanical stability, although optimal perfusion was performed to provide sufficient bone consolidation with only a large allograft. Research in the field of tibial bone defects [15,16,17,18,19,20] has shown that this procedure confers the advantage of a low morbidity rate related to the donor site [21,22,23,24]. In acute post-traumatic bone defects of the distal femur, reconstruction with free fibula transfer combined with an allograft could also be performed [25]. In this case series, the time until weightbearing was, on average, 6 month after reconstruction. However, the suitability of a vascularized fibula graft to bridge long bone defects is limited. The main disadvantage of this procedure is related to the restricted mechanical capacity of the transplant for the reconstruction of long bones with a larger diameter [26]. Moreover, because stabilising the fibula segment with an intramedullary nail is not feasible, an external fixator or osteosynthesis plate is required, which is associated with its own complication rate [26,27].
Therefore, the idea was born to manufacture a vascularized bone graft for long bone reconstruction with the method of callus distraction in a new way. The difference between this method and the usual practice of the longitudinal direction of distraction is distraction across the axis of the long bone shaft. In this method, distraction will be achieved with an expansion mode, because the device for the distraction of the callus is located in the bone marrow canal of the donor bone. The goal of this procedure is the growth of an autologous long bone vascilarized graft with an inner diameter of at least 13 mm to achieve osteosynthesis of the reconstructed long bone area with an intramedullary nail. If such a graft was available for long bone segment reconstruction, it would enable adequate surgical resection in tumor and infection cases, more or less independent of the size of the defect and additionally with the expectation of early and comfortable weightbearing. This is why this study was initiated: to design an implant prototype for an animal test in preparation for further human application.
The objective of our in vitro study was to characterize the feasibility of developing an implant prototype for fibula expansion in an in vitro model using a porcine fibula. Therefore, both the new surgical concept and a new implant prototype developed for subsequent animal testing were introduced, and the results of biomechanical testing were demonstrated.
2. Materials and Methods
The principle of an expandable device within the intramedullary canal of a longitudinally split fibula is to provide bony expansion, creating a self-perfused autologous long bone graft for segmental defects. Combining the principles of callus distraction and vascularized free fibula transfer, we aimed at a procedure that would obtain a stable autologous fibula graft with a specific inner diameter for intramedullary nail fixation. In order to provide a proof-of-concept study before conducting animal studies, a porcine in vitro model was chosen because only pigs have a fibula bone. With this model, the surgical approach and the feasibility of the concept of expanding a longitudinally split fibula needed to be demonstrated. To achieve this, we applied the callus distraction procedure to a bone expansion technique where instead of lengthening the bone, the diameter of the medullary cavity was extended with an intramedullary implant, lifting an osteotomized fibular bone fragment (Figure 1). Therefore, the porcine fibula was surgically exposed from a lateral approach. Via a drilled hole on the proximal part of the fibula, the bone marrow canal was opened. With an oscillating saw, the middle part of the fibula was split into two halves, preserving the surrounding soft tissue to retain perfusion. After lifting the created bony shell, a deflated balloon of the implant was placed via the drilled hole from the proximal end of the fibula into the medullary canal of the split fibula in the gap between one half of the fibula shaft and the bony shell. After a consolidation period, a vascularized pediculated bone graft with a similar inner diameter as the recipient bone was available, and transplantation of the graft could be performed [28]. Compared to the callus distraction method, this procedure avoids the pin tract infections associated with external fixation and enables a shorter treatment duration [29]. In order to demonstrate the feasibility of this procedure, a completely implantable medical device for fibula expansion (Figure 2) in a domestic pig model was developed and is characterized in the present study.
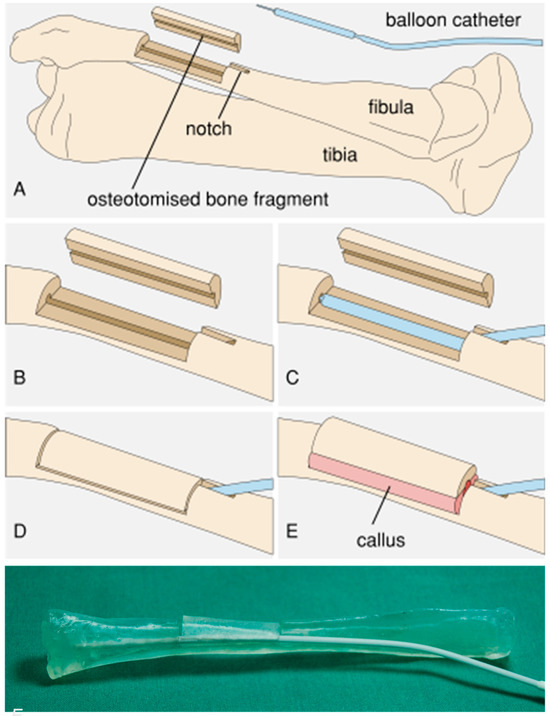
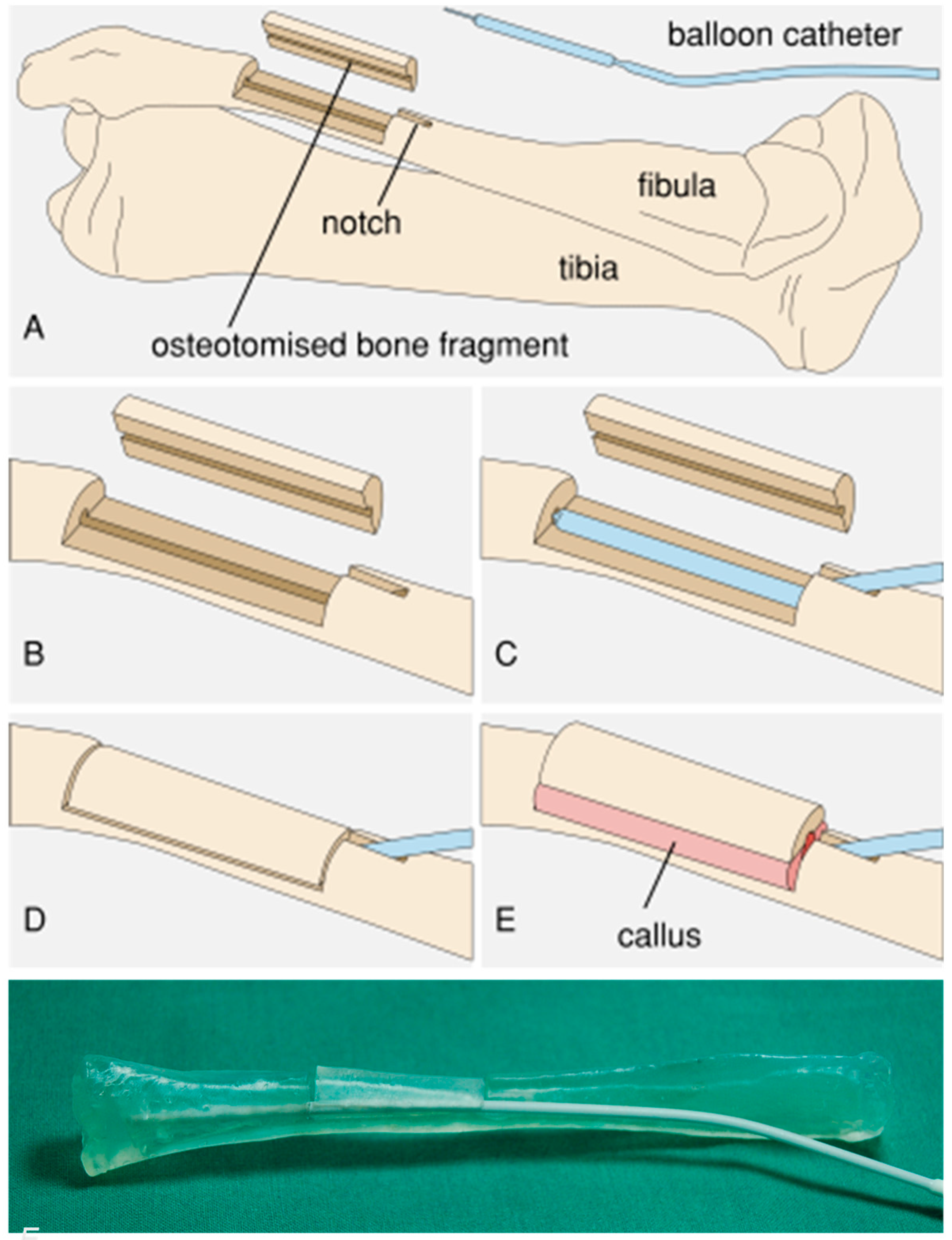
Figure 1.
Principle of the fibula expansion procedure. (A) Overview. (B) A cortical cover is prepared from a segment of the fibula and a notch is cut in the fibula. (C) The balloon catheter is implanted. (D) The cortical cover is repositioned and the wound is closed. (E) After callus formation, expansion occurs due to continuous elevation of the cortical cover via expansion of the balloon. (F) Photograph of the test setup with a 3D-printed porcine fibula model.
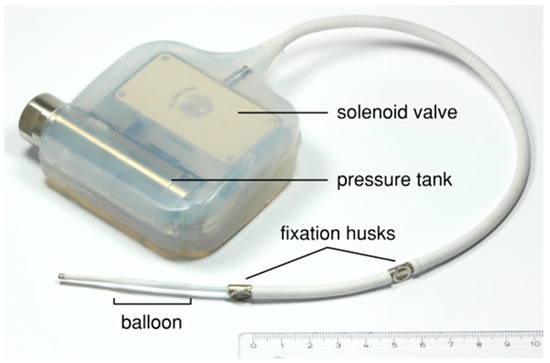
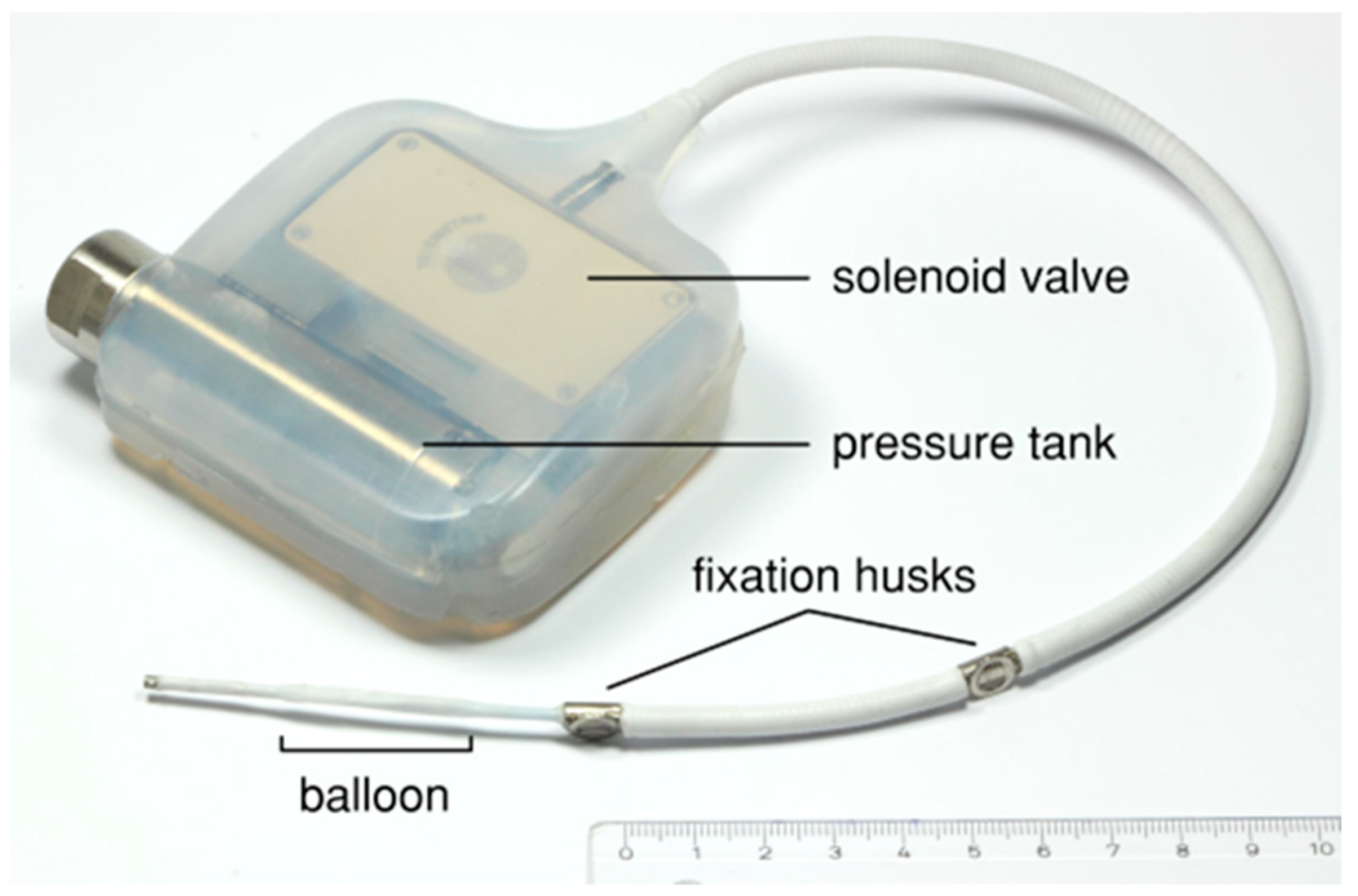
Figure 2.
Prototype of the implant device consisting of a liquid-filled pressure tank and an electronically controlled solenoid valve embedded in silicone and connected to a modified balloon catheter.
The main components of the implantable device (Figure 2) for the animal test are a liquid-filled pressure tank and a balloon catheter connected by a solenoid valve, which is controlled using a microcontroller. A commercially available balloon catheter for percutaneous transluminal angioplasty (Renma®; Terumo Corporation, Tokyo, Japan) was modified to make it suitable for the expansion of fibular bone. The balloon catheter was equipped with a titanium wire in the distal section and a high-grade steel coil around the proximal section in order to prevent breakage. A 0.25 mm thick silicone sheath (MED-4765; NuSil Technology LLC, Carpinteria, CA, USA) protects the balloon catheter against the sharp-edged bone tissue. In addition, an eyelet and two wing clamps are applied to enable the fixation and relieve strain at the distal end of the catheter. The balloon catheter has an initial diameter of about 2.4 mm and a maximum expansion diameter of 5 mm, which is adapted to the medullary cavity of the porcine fibula, with an average diameter of approximately 2 mm.
Aside from the balloon catheter, a solenoid valve is the other core component of the device. The solenoid valve acts as a breaker for fluid flow between the pressure tank and the balloon catheter. The opening of the solenoid valve is electronically controlled with a custom-made control software, created by C.M. specifically for this device (v.1). During the first seven days after implantation, the valve remains closed to enable neocallus formation. Afterwards, the balloon is gradually expanded over 2.6 days, i.e., the activated control software enables a brief opening every 20 min.
To test of the suitability of the animal implant device, a test setup was developed to simulate the in vivo situation in a domestic pig (Figure 3), providing a realistic environment for the investigation of the modified balloon catheter. Thus, the fibula and the osteotomized bone fragment were replicated with a 3D-printed porcine fibula made of polylactic acid filaments by means of the FDM 3D-printer “Replicator 2” (MakerBot, New York, NY, USA), while callus tissue was simulated by Parafilm® closure film (Pechiney Plastic Packaging, Inc., Chicago, IL, USA) which was bound to the bone surface using Loctite 4011 (Henkel AG & Co. KGaA, Düsseldorf, Germany). After the implantation and evacuation of the balloon catheter, the catheter was filled periodically with 0.05 mL sodium chloride solution every 4 min using a peristaltic pump. System pressure was measured using a pressure gauge. The amount of pressure was recorded shortly after and before each filling step. Simultaneously, the displacement of the bone fragment was recorded with an optical measuring system (Aramis; GOM GmbH, Braunschweig, Germany) to determine the pressure required to lift the osteotomized bone fragment.
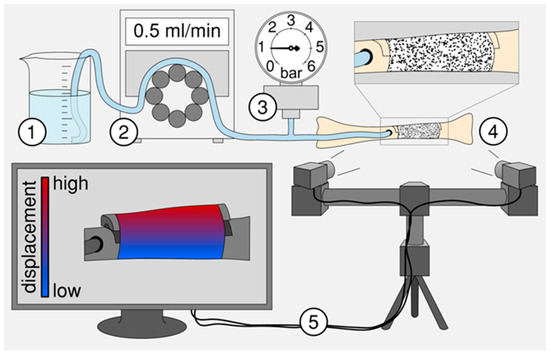
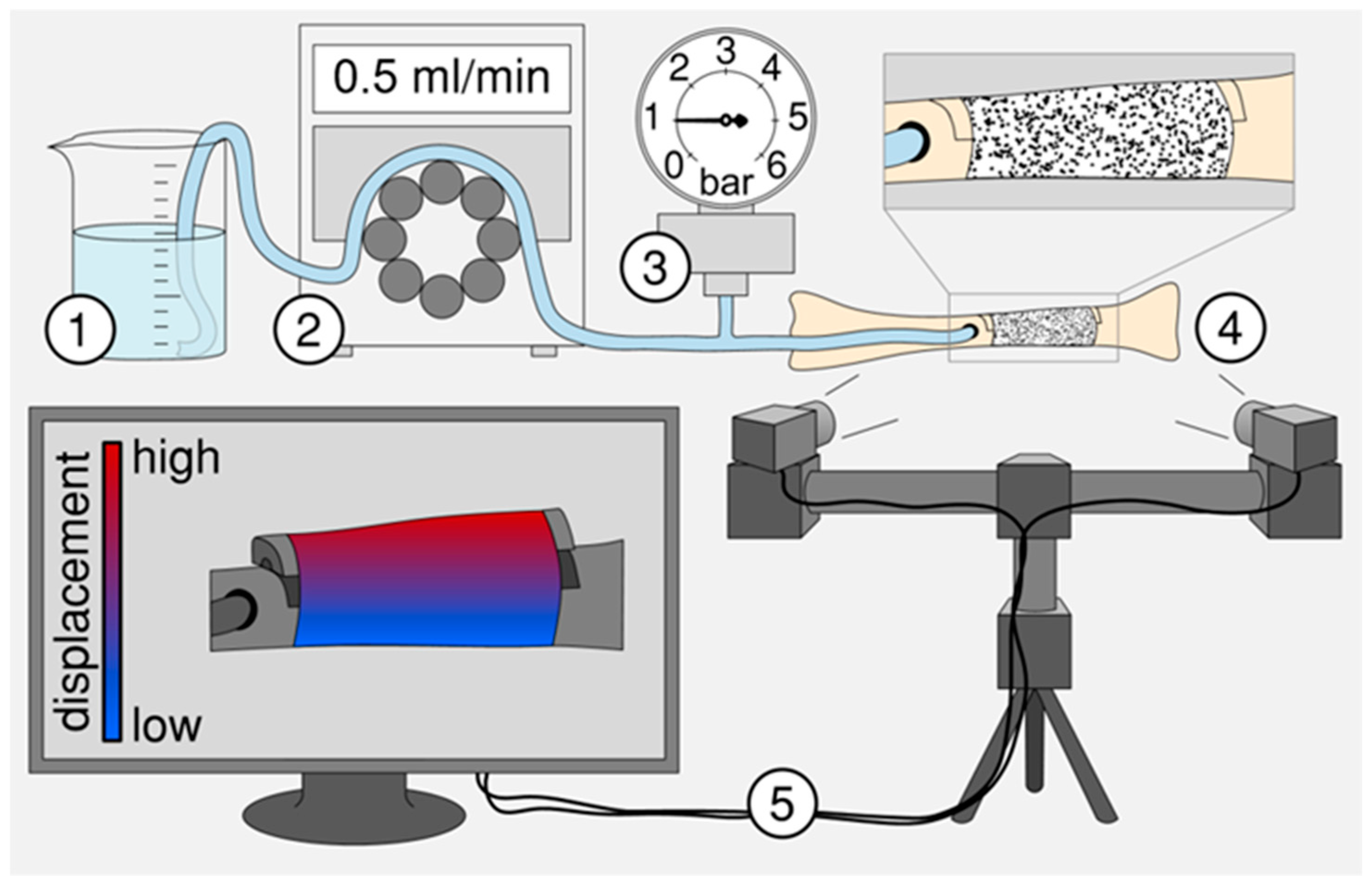
Figure 3.
Schematic of the experimental test setup to examine the suitability of balloon catheters for subsequent animal testing. The experimental setup consists of (1) a fluid reservoir, (2) a peristaltic pump, (3) a pressure gauge [30], (4) a replicated and osteotomized fibula from a domestic pig and (5) an optical measuring system to detect the displacement or elongation of the artificial callus during stepwise balloon filling.
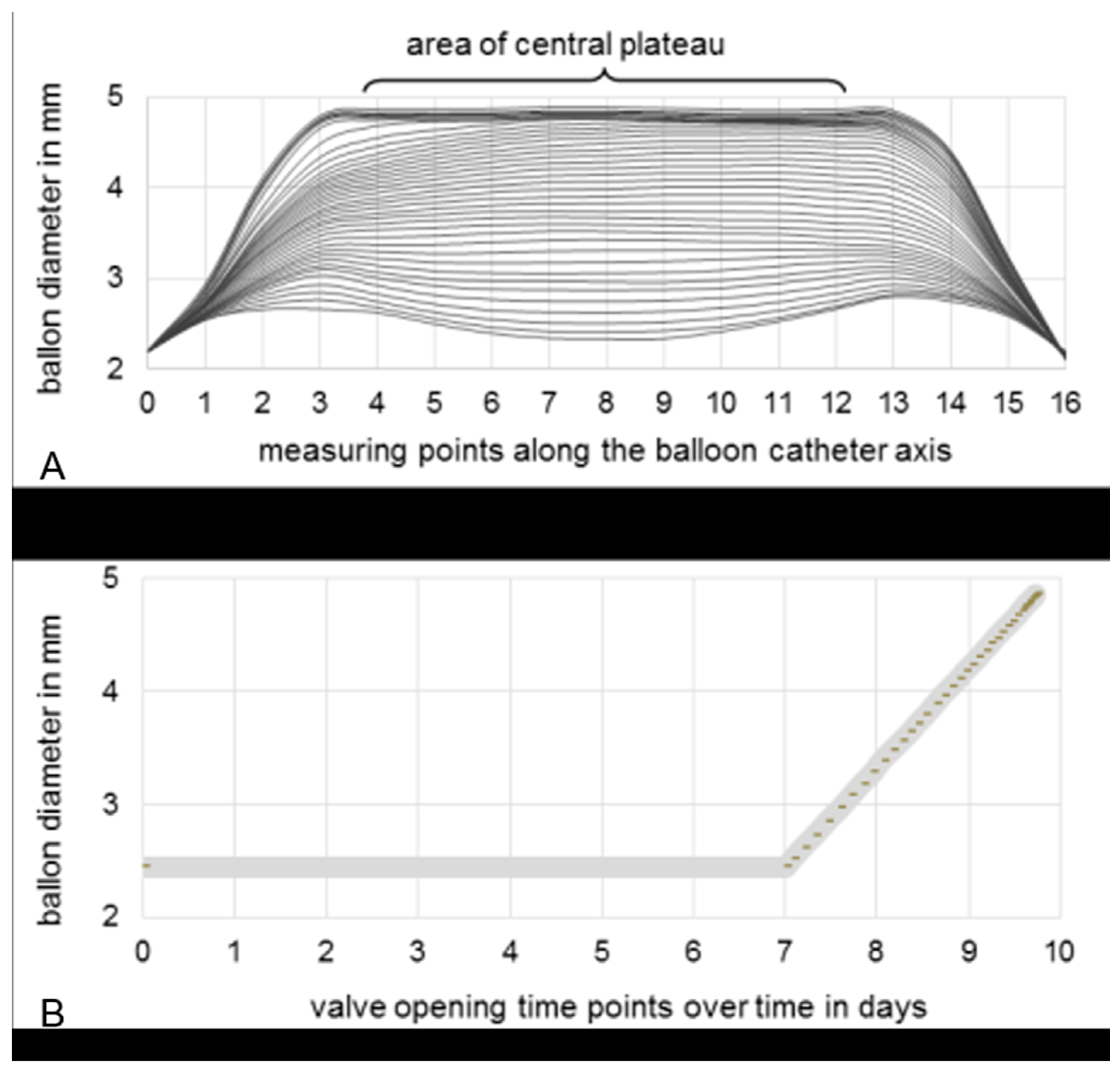
A further test was performed to analyse the set constant balloon expansion of 1 mm per day using a tempered chamber containing Ringer’s solution. The balloon’s diameter was measured optically using a profile projector (IM 6225; Keyence Deutschland GmbH, Neu-Isenburg, Germany) while the balloon was being expanded by equidistant valve openings. After each valve opening, the balloon’s diameter was measured at 16 equidistant measuring points distributed along the catheter axis. For the central plateau region, defined as the points between measuring points 4 and 12, the diameters were averaged and related to the valve openings. The test was repeated three times. The averaged measurements were used to select the best-fitting opening points of the valve to obtain a linear slope for the diameter relative to time.
3. Results
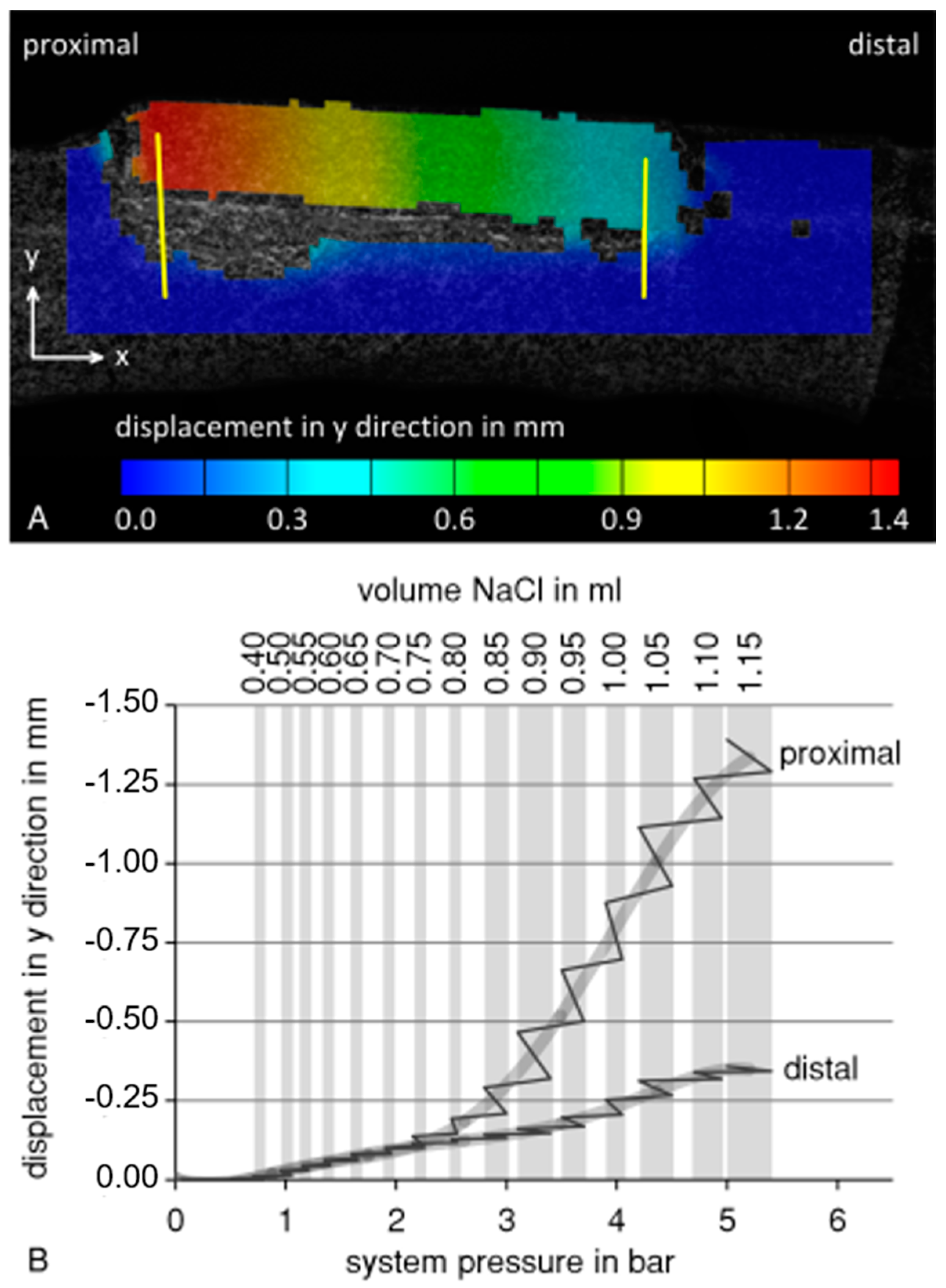
The testing of the implant prototype shows that the principle of callus distraction of the split fibula with an expandable balloon system is feasible. Data on system pressure and displacement of the bone fragment during the balloon catheter test using the 3D-printed porcine fibula are summarized in Figure 4. During balloon expansion, the osteotomized bone fragment was lifted more proximally than distally (Figure 4A). Therefore, the displacement was evaluated separately for the two sides of the bone fragment (Figure 4B). Lifting of the bone fragment began at a filling volume of 0.4 mL and system pressure of 0.8 barometric pressure. Until 0.7 mL and 1.9 barometric pressure, respectively, the proximal and distal sides were lifted homogeneously. After this point, divergence in the displacement between the proximal and distal sides of the bone fragment increased to 1.033 mm at 1.15 mL or 5 barometric pressure. As shown by the pressure and displacement measurements during the intervals with constant filling volume, system pressure decreased, while the bone fragment was still being lifted.
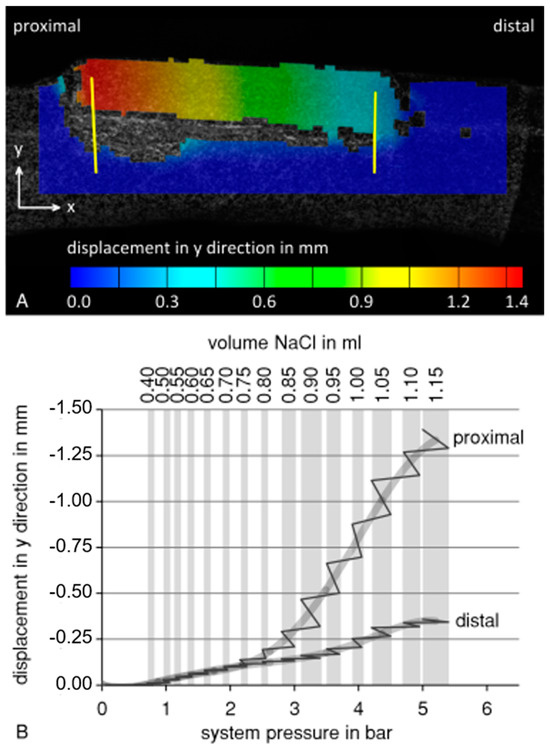
Figure 4.
Data from the balloon catheter test using a 3D-printed porcine fibula model. (A) Lifting of the bone fragment at a filling volume of 1.15 mL and 5 bar. Displacement was measured at the proximal and the distal side of the bone fragment (yellow lines) (B) Evaluation of proximal and distal displacement as a function of system pressure (solid line, grey line: averaged) during stepwise balloon filling (grey background columns: constant filling volume). During intervals with constant filling volume, system pressure within the system decreased, while the bone fragment was still being lifted.
The profile measurement of the longitudinal balloon axis during expansion is exemplarily depicted in Figure 5A. At the beginning, the diameter was inconsistent along the central plateau and at the proximal and distal ends of the balloon. After the 10th valve opening, balloon expansion was uniform until the 31st valve opening, where the maximum diameter was reached. Based on these results, almost 40 valve openings over 2.6 days were selected and set by the control software. The opening frequency increased over time, as the ratio between the volume and diameter of the cylinder was not linear and therefore the volume flow rate needed to be increased. Consequently, the balloon diameter was able to be expanded linearly over time (Figure 5B).
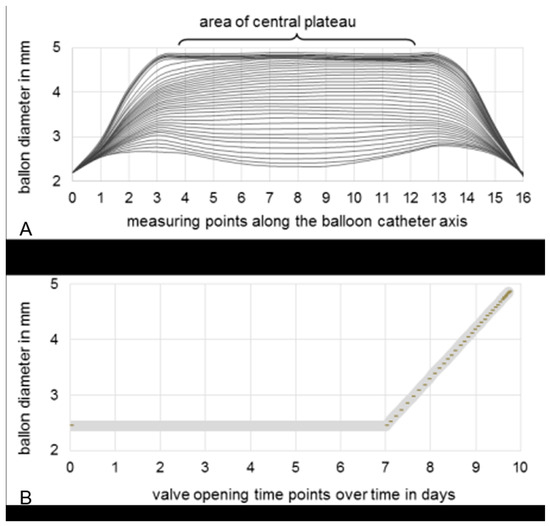
Figure 5.
(A) Profile measurement of 16 equidistant measuring points distributed along the longitudinal balloon catheter axis. Interpolated curves are based on the measured data per equidistant valve opening. (B) Selection of appropriate valve opening time-points to ensure a linear increase in balloon diameter during expansion over three days, after seven days’ waiting time to enable neocallus formation.
4. Discussion
With our fibula expansion procedure, we introduce a novel approach for the reconstruction of segmental diaphyseal bone defects, which may be associated with a shorter healing time, adequate load stability and a low risk of infection. An implant device prototype for domestic pigs was therefore developed and characterized in the present study to verify the suitability of the concept of fibula expansion before subsequent animal testing.
The mechanical test of the balloon catheter showed that the osteotomized bone fragment was not lifted uniformly. The main reason for this is the physiological shape of the medullary cavity of the fibula bone. As the proximal section of the osteotomized medullary cavity has a smaller diameter than the distal section, the lifting of the bone fragment on the proximal side occurred earlier than on the distal side.
Thus, the artificial callus material on the proximal side was subjected to a higher load, undergoing plastic deformation, even with a relatively low filling volume. In order to prevent the rupture of the newly formed callus tissue on the proximal side and the premature consolidation on the distal side in vivo, distraction speed should be approximately consistent along the bone fragment. In order to achieve this, the medullary cavity could be extended to a uniform diameter before implanting the balloon catheter, or the shape of the balloon could be adapted. Indeed, the balloon catheter test showed that the elevated system pressure led to a time-delayed plastic deformation of the artificial callus material during the intervals with a constant filling volume. Consequently, the stress applied to the callus material would be gradually decreased, thereby avoiding the rupture of the callus. When callus expansion is performed in vivo, a stress reduction within the living callus can be expected due to the growth and relaxation of the biological tissue [4,11,31].
To ensure constant callus distraction of 1 mm per day, balloon diameter needs to increase linearly over time. Based on balloon profile measurements, suitable opening time-points of the electronically controlled solenoid valve have to be selected and set by the control software. In further experimental tests with porcine specimens, the balloon catheter could be applied and lifting of the fibula bone fragment could be achieved.
A limitation of our study is that the data are limited to an in vitro model. Further research on living organisms is needed to confirm the effectiveness and safety of the new surgical concept. Furthermore, we only present exemplary results of balloon catheter expansion. The system’s development at this point requires the separate measurement of each implant device prototype because their expansion behaviors differ due to production variability, resulting in different catheter lengths and pressure tank capacities. However, after programming the individual settings with the custom-made software, a uniform expansion behavior of the balloon catheter and the fibula bone fragments can be achieved.
Furthermore, so far, we do not know the potential variabilities in bone tissue response to the expansion of the balloon catheter in an in vivo environment, nor what variations may occur in the resistance of callus tissue, as variations in the healing response in vivo may influence its expansion behavior. Therefore, during in vivo application of the device, an additional measurement and control of the displacement of the bone fragment and callus formation could be more beneficial than the detection of system pressure. During in vivo application, various problems that we attempted to minimize may occur. For example, an undetected sneaking leakage of the catheter/balloon or technical valve failures may affect the experimental measurements. To prevent the dislocation of the balloon/catheter, the tip of the catheter may be fixed with a suture. Also, in the proximal part of the balloon, which prevents the entrance of the catheter into the medullary canal of the split fibula, the catheter can be fixed with additional fixation husks and sutures. To prevent the breakage of the catheter, the inlet area was covered with a metal coil embedded in silicon wrap.
5. Conclusions
In summary, our novel implantable device should be further optimized and must be examined in future animal experiments to illustrate and prove the feasibility of this new surgical procedure and therefore of the new implant. Once there is evidence of a successful procedure in an animal model, in later clinical implementation and studies, the expansion of fibular callus tissue could be used in living human organisms to manufacture a long bone graft for tissue/bone transfer and reconstruction.
6. Patents
European patent: 12 74 6306.5; publication number: 2744433; German patent: 50 2012 006 519.4.
Author Contributions
M.M. conceived the idea for a suitable implant system and was in charge of clinical consulting. All authors developed the concept of a fibula expansion implant for an animal experiment. R.B. (Rainer Bader) and C.M. sought funding for the project from the German Federal Ministry for Economic Affairs and Energy. R.B. (Robert Bialas) and C.M. were responsible for the technical conception, development and production of the prototype and performed the experiments on the valve. J.M., C.G. and R.B. (Rainer Bader) performed the experiments with the balloon catheter plus the analysis and interpretation of the experimental data. M.M., J.M. and C.G. wrote the manuscript. R.B. (Rainer Bader), R.B. (Robert Bialas), V.B. and W.M. revised the manuscript critically. All authors have read and agreed to the published version of the manuscript.
Funding
This cooperation project was funded by the Central Innovation Program for Small and Medium-sized Enterprises (ZIM) supported by the German Federal Ministry for Economic Affairs and Energy (project numbers: KF2100704AK2 and KF2217904AK2).
Institutional Review Board Statement
At the current stage of the reported investigation, ethical approvals or consent statements are not necessary.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Matthias Militz is a consultant for BGHW (Germany) and shareholder of sBone GmbH (Germany). Christoph Miethke is a shareholder of Christoph Miethke GmbH & Co. KG. Robert Bialas is an employee of B. Braun Miethke GmbH & Co. KG. (Senior Analyst for Standards and Integration at B.Braun Miethke GmbH & Co. KG). The authors declare that they have no competing interests.
References
- Paley, D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin. Orthop. Relat. Res. 1990, 250, 81–104. [Google Scholar] [CrossRef]
- Schuh, R.; Panotopoulos, J.; Puchner, S.E.; Willegger, M.; Hobusch, G.M.; Windhager, R.; Funovics, P.T. Vascularised or non-vascularised autologous fibular grafting for the reconstruction of a diaphyseal bone defect after resection of a musculoskeletal tumour. Bone Jt. J. 2014, 96, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Spiegl, U.; Friederichs, J.; Patzold, R.; Militz, M.; Josten, C.; Buhren, V. Risk factors for failed two-stage procedure after chronic posttraumatic periprosthetic hip infections. Arch. Orthop. Trauma Surg. 2013, 133, 421–428. [Google Scholar] [CrossRef]
- Giebel, G. Callus Distraction: Clinical Application, 1st ed.; Georg Thieme Verlag: New York, NY, USA, 1992. [Google Scholar]
- Glatzel, U.; Heppert, V.; Wentzensen, A. Kallusdistraktion. Trauma Berufskrankh 2002, 4, 404–412. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, T.; Zheng, Y.; Feng, S.; Ma, X.; Zhao, F. Tibial lengthening over an intramedullary nail in patients with short stature or leg-length discrepancy: A comparative study. Int. Orthop. 2012, 36, 179–184. [Google Scholar] [CrossRef]
- Hankemeier, S.; Pape, H.C.; Jagodzinski, M.; Krettek, C. Surgical technique for callus distraction. Unfallchirurg 2004, 107, 961–964. [Google Scholar] [CrossRef]
- Jain, S.; Harwood, P. Does the use of an intramedullary nail alter the duration of external fixation and rate of consolidation in tibial lengthening procedures? A systematic review. Strateg. Trauma Limb Reconstr. 2012, 7, 113–121. [Google Scholar] [CrossRef]
- Hankemeier, S.; Bastian, L.; Gosling, T.; Krettek, C. Principles of callus distraction. Unfallchirurg 2004, 107, 945–958, quiz 959. [Google Scholar] [CrossRef] [PubMed]
- Aldegheri, R.; Renzi-Brivio, L.; Agostini, S. The callotasis method of limb lengthening. Clin. Orthop. Relat. Res. 1989, 137–145. [Google Scholar] [CrossRef]
- Ilizarov, G.A. The principles of the Ilizarov method. Bull. Hosp. Jt. Dis. Orthop. Inst. 1988, 48, 1–11. [Google Scholar]
- Tsuchiya, H.; Tomita, K.; Minematsu, K.; Mori, Y.; Asada, N.; Kitano, S. Limb salvage using distraction osteogenesis. A classification of the technique. J. Bone Jt. Surg. Br. 1997, 79, 403–411. [Google Scholar] [CrossRef]
- Zeckey, C.; Ehrl, D.; Kammerlander, C.; Bocker, W.; Neuerburg, C. All internal segmental transport in tibial bone defects: First experiences with the PRECICE bone transport system. Unfallchirurg 2020, 123, 816–821. [Google Scholar] [CrossRef]
- Capanna, R.; Bufalini, C.; Campanacci, M. A New Technique for Reconstructions of Large Metadiaphyseal Bone Defects A Combined Graft (Allograft Shell plus Vascularized Fibula). Orthop. Traumatol. 1993, 2, 159–177. [Google Scholar] [CrossRef]
- Agiza, A.R. Treatment of tibial osteomyelitic defects and infected pseudarthroses by the Huntington fibular transference operation. J. Bone Jt. Surg. Am. 1981, 63, 814–819. [Google Scholar] [CrossRef]
- Date, A.S.; Solanki, S.B.; Badhe, N.P.; Sonsale, P.D.; Pandit, H.G. Management of gap non-union of tibia by tibialisation of ipsilateral vascular fibula. J. Postgrad. Med. 1996, 42, 109–111. [Google Scholar] [PubMed]
- Hertel, R.; Pisan, M.; Jakob, R.P. Use of the ipsilateral vascularised fibula for tibial reconstruction. J. Bone Jt. Surg. Br. 1995, 77, 914–919. [Google Scholar] [CrossRef]
- Hierner, R.; Stock, W.; Wood, M.B.; Schweiberer, L. Vascularized fibula transfer. A review. Unfallchirurg 1992, 95, 152–159. [Google Scholar] [PubMed]
- Khira, Y.M.; Badawy, H.A. Pedicled vascularized fibular graft with Ilizarov external fixator for reconstructing a large bone defect of the tibia after tumor resection. J. Orthop. Traumatol. 2013, 14, 91–100. [Google Scholar] [CrossRef]
- Noaman, H.H. Management of upper limb bone defects using free vascularized osteoseptocutaneous fibular bone graft. Ann. Plast. Surg. 2013, 71, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Bodde, E.W.; de Visser, E.; Duysens, J.E.; Hartman, E.H. Donor-site morbidity after free vascularized autogenous fibular transfer: Subjective and quantitative analyses. Plast. Reconstr. Surg. 2003, 111, 2237–2242. [Google Scholar] [CrossRef]
- Ganel, A.; Yaffe, B. Ankle instability of the donor site following removal of vascularized fibula bone graft. Ann. Plast. Surg. 1990, 24, 7–9. [Google Scholar] [CrossRef]
- Imran, Y.; Zulmi, W.; Halim, A.S. Early complication following long bone reconstruction using vascularised fibula graft. Med. J. Malays. 2004, 59 (Suppl. F), 35–38. [Google Scholar]
- Maurer-Ertl, W.; Glehr, M.; Friesenbichler, J.; Sadoghi, P.; Wiedner, M.; Haas, F.; Leithner, A.; Windhager, R.; Zwick, E.B. No adverse affect after harvesting of free fibula osteoseptocutaneous flaps on gait function. Microsurgery 2012, 32, 364–369. [Google Scholar] [CrossRef]
- Venkatramani, H.; Sabapathy, S.R.; Dheenadayalan, J.; Devendra, A.; Rajasekaran, S. Reconstruction of post-traumatic long segmwent bone defects of the lower end of the femur by free vascularized fibula combined with allograft (modified Capanna’s technique). Eur. J. Trauma Emerg. Surg. 2015, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kuhner, C.; Simon, R.; Bernd, L. Vascularized fibula transplantation in orthopedic oncology. Personal experience and review of the literature. Orthopade 2001, 30, 658–665. [Google Scholar] [CrossRef]
- Le Thua, T.H.; Pham, D.N.; Boeckx, W.; De Mey, A. Vascularized fibular transfer in longstanding and infected large bone defects. Acta Orthop. Belg. 2014, 80, 50–55. [Google Scholar] [PubMed]
- Militz, M.; Oehlbauer, M. Expansion Device for Bone Expansion and Medical Device for Bone Expansion. U.S. Patent No. 9,839,461, 2012. [Google Scholar]
- Militz, M.; Gabler, C.; Mauck, J.; Miethke, C.; Bialas, R.; Buehren, V.; Mittelmeier, W.; Oehlbauer, M.; Bader, R. Rekonstruktion von Segmentdefekten der langen Röhrenknochen Indikation-Verfahren-Alternativen. Trauma Berufskrankh 2016, 18, 585–591. [Google Scholar] [CrossRef][Green Version]
- Edlich, R.F.; Winters, K.L.; Woodard, C.R.; Britt, L.D.; Long, W.B., 3rd. Massive soft tissue infections: Necrotizing fasciitis and purpura fulminans. J. Long Term Eff. Med. Implant. 2005, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.H.; Cunningham, J.L.; Kenwright, J. The forces which develop in the tissues during leg lengthening. A clinical study. J Bone Jt. Surg. Br. 1996, 78, 979–983. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).