Abstract
Background: The aim of this study was to identify the incidence of early mechanical failure in the first post-surgical year in patients who had undergone spinal surgery and to assess the related risk factors. Methods: A retrospective observational study was conducted examining all patients who consecutively underwent arthrodesis surgery. The incidence of postoperative mechanical failure during the first year was calculated as the primary outcome. Results: A total of 237 patients were identified for statistical analysis. The median age of the group of patients was 47 years (IQR of 44), and 66.6% were female. The incidence of mechanical failure in the first postoperative year was 5.1% overall, with 12 events, and the median time between surgery and the need for revision surgery was 5 months (IQR = 7.75). ASA score (OR = 2.39; p = 0.134), duration of the surgical procedure (OR = 1.27; p = 0.118), and inability to walk at discharge (OR = 7.86; p = 0.007) were independent risk factors associated with the mechanical failure. Conclusions: A higher ASA score and longer duration of surgery were risk factors for mechanical failure in the first year in patients who had undergone spinal surgery and must be carefully considered when planning spinal surgery. Early recovery of ambulation must be encouraged to prevent mechanical failure.
1. Introduction
In recent decades, thanks to improvements in the surgical techniques and instrumentation available, there has been a considerable increase in the use of spinal fusion surgery for the treatment of degenerative spinal pathologies [1,2].
In recent decades, the focus of instrumented spinal surgery has shifted from a method to correct deformities to a method to restore stability and maintain natural balance. The development of materials and techniques has evolved, and today, fixation systems can include different modalities and techniques. The surgeon may approach the spine from the back (posterior), the front (anterior), or the side (lateral). The approach depends on the level of the spine that will be fused and other factors [1].
Such surgery seeks to limit the movement of spinal segments that cause pain after spinal fusion. The main pathologies in which this procedure is indicated are adult scoliosis, kyphosis, disc herniation, vertebral fractures, spinal stenosis, and spondylolisthesis [3,4]. Spinal fusion is surgery to connect two or more bones in any part of the spine using metal plates, screws, or rods that might hold the bones together.
The outcomes following such procedures, which often vary widely in terms of the type and number of vertebrae involved, are not clear and, to date, no true postoperative care best practice has been identified to achieve the best results. A survey conducted in two European countries showed that the care provided in the preoperative, operative, and postoperative phases is provided very differently for patients undergoing lumbar spine fusion [5].
In the postoperative period, a significant number of patients may experience postoperative mechanical complications such as implant breakage and proximal or distal junctional syndrome, i.e., degeneration of the vertebral segment adjacent to the stabilized district.
This condition often requires revision surgery with intra- and peri-operative complication risks of 18% and 39%, respectively [6]. Proximal junctional failure and rod fracture are cited by Yasuda et al. [7] as the main causes of revision surgery following a mechanical failure problem, although the risk factors for this event should not be looked for in mechanical problems alone. In a 2021 systematic review, Noh et al. reported an overall incidence of rod breakage of 12%, with a variability from 7 to 18% [8]. Such an event has a negative impact on the patient’s health and often makes new instrumental revision necessary [9,10]. Mohi Eldin et al. reported that, in procedures using pedicle screws in the lumbar region, mechanical failure occurs early, within the first six months after surgery [11]. Therefore, an understanding of the relative risk factors associated with the mechanical failure of the systems used is increasingly important [12,13,14]. The most frequently investigated risk factors are related to patient demographics and radiological and surgical techniques [15,16,17]. Old age, a higher body mass index (BMI), prior spinal surgery, and pedicle subtraction osteotomy were the risk factors identified by Noh et al. [8].
Early recovery of ambulation after such surgeries is encouraged in clinical practice [18], but is not always an easy goal to achieve. To date, a ‘best practice’ for the post-surgical treatment of patients undergoing larger arthrodesis has not been defined and the rehabilitation approach appears to be implemented very heterogeneously [19]. Currently, early mobilization programs—understood as transfer and ambulation training—are playing an increasing role in managing pain symptoms and preventing complications after spinal surgery [20,21,22]. In contrast, the role of such recovery in relation to possible mechanical failure in the first year after surgery is poorly described in the literature.
The aim of this study was to identify the incidence of early mechanical failure in the first post-surgical year in patients who had undergone spinal surgery for degenerative disease and to assess the related risk factors.
2. Materials and Methods
2.1. Study Design
The article is designed as a retrospective observational study.
2.2. Setting and Patients
This study was conducted at a spinal surgery unit of an Italian single-specialty orthopedic hospital, examining all patients who consecutively underwent surgery between March 2018 and March 2019. A computerized system was used to identify the patients who could be possible candidates for the study. The inclusion criteria were: patients who had undergone, for the first time, spinal arthrodesis for degenerative disease involving the use of some form of instrumentation (rods or screws). All patients were included, irrespective of the diagnosis that led to the need for such surgery. The exclusion criteria were: patients who had undergone a spinal procedure without the application of any instrumentation—i.e., herniectomy, vertebroplasty, and laminectomy—and subjects admitted for mechanical dysfunction and undergoing revision surgery for a previous failed surgery.
The study was approved by the Ethics Committee with the protocol number 0007166 and was registered on the Clinicaltrials.gov database (NCT04983576). Last data access is on 1 March 2024.
2.3. Surgical Procedure
The posterior arthrodesis procedure consisted of applying a high screws density inside the pedicles, which were then connected with a rod, and securing nuts to ensure the implant’s biomechanical tightness and promote vertebral fusion. The screw size was 6.0 mm and 2 rods were used in the construction. Based on the clinical evaluation and biomedical imaging, a team of experienced orthopedic surgeons determined the most appropriate surgical modalities, such as the number of levels to be stabilized and the best surgical approach (screw size and number of rods). The surgical procedure was discussed and planned by orthopedic surgeon teams that were the same for all patients who had undergone surgery enrolled in the study. This aspect ensured a similar surgical approach for all the enrolled patients.
2.4. Postoperative Care and Rehabilitation
In the postoperative phase, the patients were followed by a multidisciplinary team of orthopedic surgeons, physiatrists, nurses and physiotherapists. This team was tasked with discussing any problems that may have arisen during the course of postoperative treatment and monitoring of the patient’s recovery, establishing the timing of discharge and any orthosis prescription. On the basis of the patient’s clinical condition and home care network, the post-discharge care pathway was decided. Before discharge from the hospital, X-rays were checked.
Physiotherapy started the day after surgery and included two exercise sessions per day, Monday through Friday, and an additional session on Saturday morning. Each physiotherapy session lasted 30 min and was delivered by a physiotherapist experienced in orthopedic spine surgery. The aim of rehabilitation was to recover basic autonomy and early walking. The physiotherapy session included two steps. The first step included bed exercises performed independently by the person and an assessment of muscular or sensory deficit. The second step of treatment included assistance with verticalization maneuvers to sit up and stand upright, walking, and training on climbing stairs. Furthermore, the physiotherapist instructed on postures and movements allowed or not. The patient was encouraged to repeat the learned exercises independently, to get out of bed, and to ambulate. Training was also provided to the caregiver in order to help the patient with recovery of autonomy.
The routine follow-up was 3 and 9 months after discharge from the hospital. After the first visit, the patient was free to have full mobility and physical activity. To check for bone healing, the CT scan was at 9 months.
2.5. Outcomes
The primary outcome was the incidence of postoperative mechanical failure during the first year, and was calculated as the ratio between the number of diagnoses of mechanical failure and the total number of arthrodesis procedures performed. The diagnosis of mechanical failure included the diagnosis of rod and/or screw breakage (bilateral or unilateral), vertebral fracture proximal or distal to the instrumentation, mobilization of the fixation device without breakage thereof, and junctional syndrome for which the instrumentation required revision. The diagnoses were collected by the physiotherapist in charge of the research by consulting the patient’s medical records. The diagnosis had to be made during the first year after surgery. The secondary outcome was the assessment of the time elapsed between the date of surgery and the diagnosis of mechanical failure.
2.6. Variables Collected
Possible risk factors were identified through the literature and multidisciplinary discussion among professionals (nurses, physiotherapists, physiatrist, and orthopedic surgeon) [13]. The variables collected were summarized in three groups:
- Demographic variables: age, sex, body mass index (BMI), smoking, diagnosis of diabetes, diagnosis of depression, diagnosis of osteoporosis, anesthesiologic risk defined preoperatively with the ASA score, anamnesis of previous spinal surgery but not arthrodesis, the diagnosis that led to surgery.
- Surgical and postoperative variables: number of spinal levels involved in the stabilization, involvement of the lumbosacral vertebrae, length of surgery (calculated at 15 min intervals), surgical approach (posterior and other), bone graft surgery procedure, ability to walk more than 10 m at hospital discharge without aids, time of immobilization after surgery, time between surgery and walking recovery and length of stay.
The data were collected by consulting the patients’ clinical records, available on the computer system. From each file, information was gathered starting from the day of admission to the ward up to the day of discharge and any subsequent re-admissions or outpatient visits. The research physiotherapists were in charge of collecting the data and recording them in a computer database.
2.7. Statistical Analysis
A descriptive analysis of the data was performed using the central trends, frequencies, and relative dispersion measures for the individual outcomes and variables. The relationship between the primary outcome and the other variables was investigated using an ordinal logistic regression model and the independent variables were selected applying a backward procedure, first limiting any confounding by including all variables in the model and then adjusting the effects for all the factors analyzed. The variables were then removed individually at each step, starting with those with the highest p-value. The selection process was suspended until all the variables included in the model proved significant. The level of significance was set at p < 0.157. A receiver operator characteristic (ROC) curve analysis was used to check the logistic regression model. Statistical interpretation of the data was performed using SPSS software.
A post hoc power calculation was undertaken, as a convenience sample was being used. In the unmatched cohort, assuming a type I error of 0.05, as well as the rate of primary outcome and study group size as that reported in the results, the analysis had 95% power, adequate to undertake and report on the analyses of interest. With a sample size of 250 patients eligible for inclusion in the study and with the expected incidence of mechanical problems according to data available in the literature ranging from 7 to 12%, 18 to 30 events are to be expected.
3. Results
In total, 306 patients eligible for the study were identified. Of these, 7 could not be enrolled for data loss, 26 were lost to the study as they did not show up for their follow-up visits, and 36 were admitted for mechanical dysfunction and undergoing revision surgery for a previous failed surgery. The statistical analysis was, therefore, conducted on a total of 237 patients. Figure 1 shows the relevant flow chart.
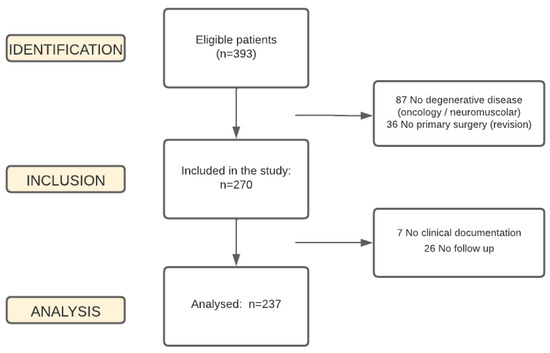
Figure 1.
Enrollment process.
The mean age of the group of patients was 47 years (IQR of 42), and 66.2% were female. The baseline characteristics and the variables gathered for all patients enrolled are summarized in Table 1.

Table 1.
Patient’s characteristics. Data were n (%) for categorical variables and median (IQR: interquartile range) for continuous variables.
The incidence of mechanical failure in the first postoperative year was 5.06% overall with 12 events. If considering implant breakage alone, the incidence was 2.2%, for screws loosening, it was 1.6%, and for junctional fracture it was 1.3%. The median time between surgery and the need for revision surgery following the diagnosis of mechanical failure was 5 months (IQR = 7.75). Among the 12 patients diagnosed with mechanical failure undergoing a first surgical revision, a second surgical revision was required for 4 patients (or 33.3%). From the multivariate analysis (Table 2), the ASA score (OR = 2.396; p = 0.134), duration of the surgical procedure (OR = 1.27; p = 0.118), and achievement of walking (OR = 7.86; p = 0.007) were the independent risk factors for mechanical failure.

Table 2.
Logistic regression for 1-year failure. Factors included according to a backward * procedure with a p-for removal fixed at 0.157.
4. Discussion
The incidence of mechanical failure in the first year after surgery was 5.06%, with the time to diagnosis of the issue from the time of surgery being a median of 5 months. This is below the overall incidence presented by Noh et al. [8] in their systematic review of the literature, which reported a rate of 12% for implant breakage alone. This difference can be explained by a much longer follow-up for the studies selected by Noh et al., where the average time to breakage was calculated to be 23.4 months after surgery.
The comprehensive data collection, the enrolment of all consecutive patients, the standardized treatment provided by the same team of healthcare professionals, and the different variables also collected regarding postoperative care were the strengths of the study and ensured its robustness, despite the low number of mechanical failures collected.
The independent risk factors for mechanical failure revealed by the multivariate analysis were ASA score, duration of surgery, and failure to recover ambulation without aids during hospitalization.
Lynch et al. [23] showed a result in the opposite direction. In patients who underwent single-level lumbar spine fusion surgery, the ASA score was not a significant risk factor for the possible complications or functional recovery of patients in the first two postoperative years. This difference in outcome could be explained precisely by the population considered. In the present study, the median number of spinal levels involved in the surgery was 5, with a range from 2 to 12, thus, with a type of surgery certainly more important than and different from the Lynch study. In line with this hypothesis, it should be noted that, also in the study by Phan et al. [24], it was shown that, for patients undergoing cervical arthrodesis, the American Society of Anesthesiologists (ASA) score could be a valuable tool in identifying patients most at risk of hospital readmission in the first 30 days after surgery. Among the independent predictors of implant-related complications identified by Soroceanu et al. [14], the ASA score was also significant.
Among the surgical variables, a longer length of the arthrodesis and a longer length of the surgical procedure were risk factors for mechanical failure. Specifically, in our study, length of surgery (calculated at 15 min intervals) increased the risk of rupture by more than twofold after a certain threshold. Several authors [16,17,25] have reported this association in the literature. Aldebayan et al. [25] reported that operations lasting at least 4 h and 19 min were associated with poorer functional outcomes at discharge, thus, it was more likely that the patient would be discharged to a healthcare facility rather than to their own homes. Demura et al. [17], in a pediatric population, identified a slightly higher cut-off with a surgery duration greater than 5 h as a predictor of postoperative complications.
A closer follow-up may be considered in patients who have a high ASA score and in those undergoing a long surgery. These patients are at a higher risk of mechanical failure and should be encouraged to be more aware of this complication and alert for possible signs and symptoms.
In the present study, other surgical variables were not found to be risk factors for mechanical failure. Based on the systematic review of Noh et al. [8], Bae [26] highlighted that fusion level, rod diameter, rod material, and change in sagittal parameters were not effective for rod fracture. Advancements in surgical planning, techniques, and surgeon experience could explain the reduction in the role of surgical procedures in causing mechanical complications [27].
Among the variables involving postoperative care, the inability to regain ambulation has proved to be a significant risk factor. The importance of the early recovery of ambulation after spine surgery has been reported by several authors [20,21]. Lovecchio et al. [22], considering a population of adults diagnosed with spinal deformity, reported, in a multivariate analysis, the recovery of ambulation as a protective factor for postoperative complications. In the present study, recovery of ambulation also played an important role in the medium and long term. It can be assumed that early verticalization provides mechanical stress on the vertebral bony tissue, thus stimulating the calcification process, and this most likely facilitates the stabilization of the instrumentation inserted during the arthrodesis procedure. The importance of loading in healing after stabilization surgery has already been highlighted in other types of interventions, such as stabilization after tibial diaphysis fracture [28,29,30,31]. Further studies on a specific population such as those undergoing spinal arthrodesis surgery are needed to better understand the role of loading in the postoperative calcification process. In addition, the possibilities and timing of ambulation recovery for these patients have not been fully explored.
In clinical practice, the data from this study seem to support the need to implement care aimed at the early recovery of ambulation without aids in all arthrodesis patients, regardless of diagnosis or the number of vertebral levels stabilized. Patients should be encouraged to walk several times a day and for progressively longer distances from the early stages of postoperative care. The data show that patients with a higher incidence of mechanical failure are also those with a longer immobilization time in bed. This indication should be taken into account when planning the postoperative course of care and implementing physiotherapy treatment. Several authors have pointed out the risk for patients to develop a reduced walking ability and less than what might be expected after lumbar spine surgery [32,33]. For more complex spinal procedures—those involving multiple spinal sections and multiple levels—the suitability of early mobilization approaches is unclear, and the factors that may play a role in delaying the early recovery of ambulation are not described.
In order to improve our knowledge about mechanical failure in the future, studies must be planned taking into account a number of elements that have emerged. Firstly, in order to achieve a consistent sample size with respect to an event with a limited incidence such as mechanical failure, the planning of multicentric studies will be encouraged. Secondly, studies should be prospective, with the possibility of prolonged follow-up over time, so that mechanical failure events occurring over time can also be intercepted. The longevity of arthrodesis implants is an aspect that will have to be investigated in order to really assess the impact of this type of intervention. Finally, not only the aspects related to surgical procedures and clinical characteristics will have to be taken into account as risk factors, but also all aspects related to lifestyle, occupation, and functional capacity, which, in the long term, may have a positive or negative impact on the longevity of the instrumentation. Certain repeated movements over time or certain stressful activities for the spine over time could be the cause of instrument failure and will have to be taken into account.
Limits: the study does have some limitations. First of all, the relatively low incidence of the primary outcome did not allow for an analysis within the individual diagnostic categories (scoliosis, discopathy, stenosis, spondylolisthesis, and others). This was not one of the objectives of this study, but on the basis of the reported incidence data for each category, further studies will be needed. The study sample size was in line with the published studies in the literature, where it ranged from 30 to 526 [8], and, according to the authors, nevertheless allowed for a consistent answer to the study’s research question. Specific studies for different types of diagnosis are necessary to verify if the results found can be generalized for all patients undergoing arthrodesis.
Secondly, this is a retrospective study and data were collected by consulting the patients’ medical records, which were available on the computer system. Clinical records and follow-up data were not available for 7 and 26 patients, respectively. The retrospective nature of the study made it impossible to provide data about patients who did not return for postoperative routine follow-up. The reasons for this choice by patients may vary, but it cannot be excluded that they were also related to postoperative complications, including mechanical failures. On the other hand, missed data were limited and the collected data were carefully checked and are comprehensive, therefore, they allowed for an adequate statistical analysis of the primary outcome.
The results of this study may form the basis for planning prospective studies. The patient’s lifestyle in the postoperative period is a factor that should be considered as a possible predictor. Repetitive bending or twisting movements of the trunk during activities of daily living or work could cause stress for the arthrodesis and facilitate mechanical failure.
5. Conclusions
Mechanical failure is an important postoperative complication after spinal arthrodesis in the first year after surgery. A higher ASA score and longer duration of surgery are risk factors for mechanical failure and must be carefully considered when planning spinal surgery. During hospitalization, the recovery of ambulation must be encouraged, showing a positive association with a reduced risk of mechanical failure. Early verticalization can put mechanical stress on the vertebral bony tissue, thus stimulating the calcification process, and this most likely facilitates the stabilization of the instrumentation inserted during the arthrodesis procedure.
All these factors will be useful for better identifying patients, and a closer follow-up is needed.
Author Contributions
Conceptualization, methodology, writing and validation: V.P. and A.C. Software, validation: A.E. Formal analysis, investigation: M.M. Data curation, writing—original draft preparation: R.R. and F.A. Review and editing, visualization, supervision: M.G.B. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Istituto Ortopedico Rizzoli (CE-AVEC 354/2021/Oss/IOR approved on 22 April 2021).
Informed Consent Statement
Not applicable because for Italian law informed consent is not required for retrospective observational studies.
Data Availability Statement
Data available on request due to restrictions, e.g., privacy or ethical. The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Abbreviations
| BMI | body mass index |
| PSO | pedicle subtraction osteotomy |
| CT | computed tomography |
| ROC | receiver operator characteristic |
| IQR | interquartile range |
References
- De Kunder, S.L.; Rijkers, K.; Caelers, I.J.M.H.; de Bie, R.A.; Koehler, P.J.; van Santbrink, H. Lumbar Interbody Fusion: A Historical Overview and a Future Perspective. Spine 2018, 43, 1161–1168. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Snyder, W. Spinal instrumentation with a low complication rate. Surg. Neurol. 1997, 48, 566–574. [Google Scholar] [CrossRef]
- Bennett, G.J.; Serhan, H.A.; Sorini, P.M.; Willis, B.H. An experimental study of lumbar destabilization. Restabilization and bone density. Spine 1997, 22, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Schnee, C.L.; Freese, A.; Ansell, L.V. Outcome analysis for adults with spondylolisthesis treated with posterolateral fusion and transpedicular screw fixation. J. Neurosurg. 1997, 86, 56–63. [Google Scholar] [CrossRef] [PubMed]
- van Erp, R.M.A.; Jelsma, J.; Huijnen, I.P.J.; Lundberg, M.; Willems, P.C.; Smeets, R.J.E.M. Spinal Surgeons’ Opinions on Pre- and Postoperative Rehabilitation in Patients Undergoing Lumbar Spinal Fusion Surgery: A Survey-Based Study in the Netherlands and Sweden. Spine 2018, 43, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.; Langella, F.; Mazzucchelli, L.; Lamartina, C. Revision strategies for failed adult spinal deformity surgery. Eur. Spine J. 2020, 29 (Suppl. 1), 116–125. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Yamato, Y.; Hasegawa, T.; Yoshida, G.; Banno, T.; Arima, H.; Oe, S.; Mihara, Y.; Ide, K.; Matsuyama, Y. Revision Surgery Due to Proximal Junctional Failure and Rod Fracture in Adult Deformity Surgery at a Single Institution in Japan. Spine Surg. Relat. Res. 2022, 6, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.H.; Kim, K.H.; Park, J.Y.; Kuh, S.U.; Kim, K.S.; Cho, Y.E.; Chin, D.K. Characteristics and Risk Factors of Rod Fracture Following Adult Spinal Deformity Surgery: A Systematic Review and Meta-Analysis. Neurospine 2021, 18, 447–454. [Google Scholar] [CrossRef]
- Glassman, S.D.; Bazzi, J.; Puno, R.M.; Dimar, J.R. The durability of small-diameter rods in lumbar spinal fusion. J. Spinal Disord. 2000, 13, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Shaffrey, C.I.; Ames, C.P.; Demakakos, J.; Fu, K.M.; Kes-havarzi, S.; Li, C.M.Y.; Deviren, V.; Schwab, F.J.; Lafage, V.; et al. Assessment of symptomatic rod fracture after posterior instrumented fusion for adult spinal deformity. Neurosurgery 2012, 71, 862–867. [Google Scholar] [CrossRef]
- Mohi Eldin, M.M.; Ali, A.M. Lumbar transpedicular implant failure: A clinical and surgical challenge and its radiological assessment. Asian Spine J. 2014, 8, 281–297. [Google Scholar] [CrossRef]
- DeWald, C.J.; Stanley, T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients overage 65: Surgical considerations and treatment options in pa-tients with poor bone quality. Spine 2006, 31 (Suppl. 19), S144–S151. [Google Scholar] [CrossRef] [PubMed]
- Masferrer, R.; Gomez, C.H.; Karahalios, D.G.; Sonntag, V.K. Efficacy of pedicle screw fixation in the treatment of spinal instability and failed back surgery: A 5-year review. J. Neurosurg. 1998, 89, 371–377. [Google Scholar] [CrossRef]
- Soroceanu, A.; Diebo, B.G.; Burton, D.; Smith, J.S.; Deviren, V.; Shaffrey, C.; Kim, H.J.; Mundis, G.; Ames, C.; Errico, T.; et al. Radiographical and Implant-Related Complications in Adult Spinal Deformity Surgery: Incidence, Patient Risk Factors, and Impact on Health-Related Quality of Life. Spine 2015, 40, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Shaffrey, E.; Klineberg, E.; Shaffrey, C.I.; Lafage, V.; Schwab, F.J.; Protopsaltis, T.; Scheer, J.K.; Mundis, G.M.; Fu, K.M.; et al. Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J. Neurosurg. Spine 2014, 21, 994–1003. [Google Scholar] [CrossRef]
- Lertudomphonwanit, T.; Kelly, M.P.; Bridwell, K.H.; Lenke, L.G.; McAnany, S.J.; Punyarat, P.; Bryan, T.P.; Buchowski, J.M.; Zebala, L.P.; Sides, B.A.; et al. Rod fracture in adult spinal deformity surgery fused to the sacrum: Prevalence, risk factors, and impact on health-related quality of life in 526 patients. Spine J. 2018, 18, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Demura, S.; Ohara, T.; Tauchi, R.; Takimura, K.; Watanabe, K.; Suzuki, S.; Uno, K.; Suzuki, T.; Yanagida, H.; Yamaguchi, T.; et al. Incidence and causes of instrument-related complications after primary definitive fusion for pediatric spine deformity. J. Neurosurg. Spine 2022, 38, 192–198. [Google Scholar] [CrossRef]
- Wainwright, T.W.; Immins, T.; Middleton, R.G. Enhanced recovery after surgery (ERAS) and its applicability for major spine surgery. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 91–102. [Google Scholar] [CrossRef]
- Elsarrag, M.; Soldozy, S.; Patel, P.; Norat, P.; Sokolowski, J.D.; Park, M.S.; Tvrdik, P.; Kalani, M.Y.S. Enhanced recovery after spine surgery: A systematic review. Neurosurg. Focus 2019, 46, E3. [Google Scholar] [CrossRef]
- Huang, J.; Shi, Z.; Duan, F.F.; Fan, M.X.; Yan, S.; Wei, Y.; Han, B.; Lu, X.M.; Tian, W. Benefits of Early Ambulation in Elderly Patients Undergoing Lumbar Decompression and Fusion Surgery: A Prospective Cohort Study. Orthop. Surg. 2021, 13, 1319–1326. [Google Scholar] [CrossRef]
- Adogwa, O.; Elsamadicy, A.A.; Fialkoff, J.; Cheng, J.; Karikari, I.O.; Bagley, C. Early Ambulation Decreases Length of Hospital Stay, Perioperative Complications and Improves Functional Outcomes in Elderly Patients Undergoing Surgery for Correction of Adult Degenerative Scoliosis. Spine 2017, 42, 1420–1425. [Google Scholar] [CrossRef]
- Lovecchio, F.; Jordan, Y.; Punyala, A.; Shah, S.; Lafage, R.; Charles Elysee, J.; Sheikh, B.; Steinhaus, M.; Ang, B.; Schwab, F.; et al. Timing of inpatient medical complications after adult spinal deformity surgery: Early ambulation matters. Spine J. 2023, 23, 219–226. [Google Scholar] [CrossRef]
- Lynch, C.P.; Cha, E.D.K.; Geoghegan, C.E.; Jadczak, C.N.; Mohan, S.; Singh, K. Higher American Society of Anesthesiologists Classification Does Not Limit Safety or Improvement Following Minimally Invasive Transforaminal Lumbar Interbody Fusion. Neurospine 2022, 19, 533–543. [Google Scholar] [CrossRef]
- Phan, K.; Kim, J.S.; Lee, N.J.; Kothari, P.; Cho, S.K. Relationship Between ASA Scores and 30-Day Readmissions in Patients Undergoing Anterior Cervical Discectomy and Fusion. Spine 2017, 42, 85–91. [Google Scholar] [CrossRef]
- Aldebeyan, S.; Aoude, A.; Fortin, M.; Nooh, A.; Jarzem, P.; Ouellet, J.; Weber, M.H. Predictors of Discharge Destination After Lumbar Spine Fusion Surgery. Spine 2016, 41, 1535–1541. [Google Scholar] [CrossRef]
- Bae, J. Commentary on “Characteristics and Risk Factors of Rod Fracture Following Adult Spinal Deformity Surgery: A Systematic Review and Meta-Analysis”. Neurospine 2021, 18, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Bari, T.J.; Hallager, D.W.; Hansen, L.V.; Dahl, B.; Gehrchen, M. Reducing revision rates following Pedicle Subtraction Osteotomy surgery: A single-center experience of trends over 7 years in patients with Adult Spinal Deformity. Spine Deform. 2021, 9, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, A.; Kovoor, J.G.; Stretton, B.; Kieu, J.T.; Bright, R.A.; Hewitt, J.N.; Ovenden, C.D.; Gupta, A.K.; Afzal, M.Z.; Edwards, S.; et al. Outcomes of early versus delayed weight-bearing with intramedullary nailing of tibial shaft fractures: A systematic review and meta-analysis. Eur. J. Trauma Emerg. Surg. 2022, 48, 3521–3527. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Zhang, Z.; Zhang, C.; Feng, Y.; Zhang, S. Effect of time to first ambulation on recurrence after PELD. J. Orthop. Surg. Res. 2020, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cammisa, F.P., Jr.; Sandhu, H.S.; Girardi, F.P.; Khan, S.N. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine 2002, 27, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Gruskay, J.A.; Fu, M.; Bohl, D.D.; Webb, M.L.; Grauer, J.N. Factors affecting length of stay after elective posterior lumbar spine surgery: A multivariate analysis. Spine J. 2015, 15, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, S.J.; Hahne, A.J.; Davidson, M.; McClelland, J.A. Predictors of substantial improvement in physical function six months after lumbar surgery: Is early post-operative walking important? A prospective cohort study. BMC Musculoskelet. Disord. 2019, 20, 418. [Google Scholar] [CrossRef] [PubMed]
- Smuck, M.; Muaremi, A.; Zheng, P.; Norden, J.; Sinha, A.; Hu, R.; Tomkins-Lane, C. Objective measurement of function following lumbar spinal stenosis decompression reveals improved functional capacity with stagnant real-life physical activity. Spine J. 2018, 18, 15–21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).