Robotic Liver Resection: Report of Institutional First 100 Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
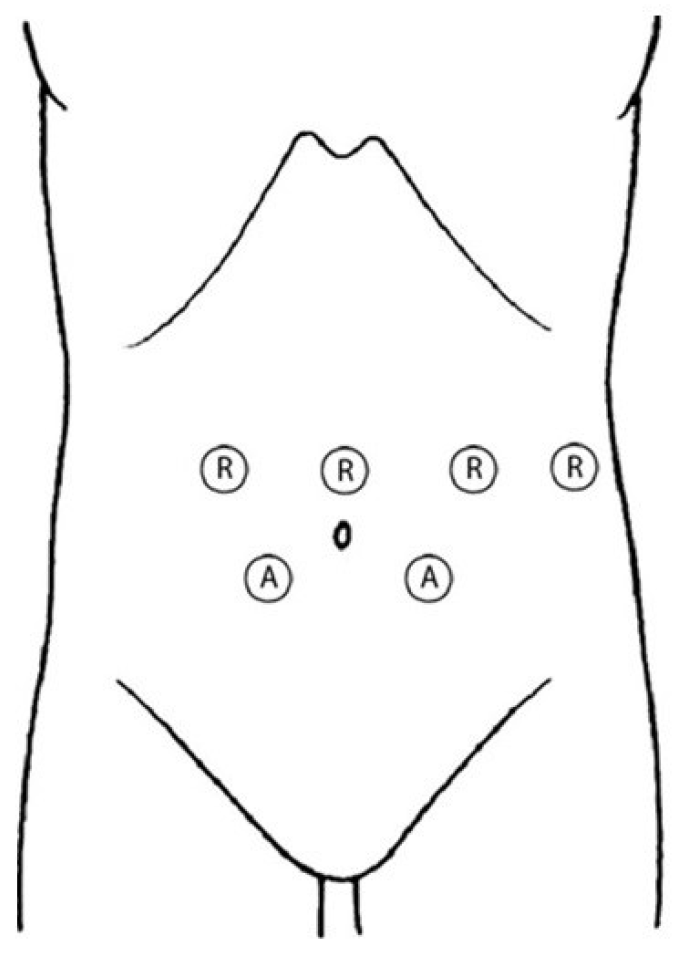
2.2. Surgical Technique
2.3. Statistical Analysis
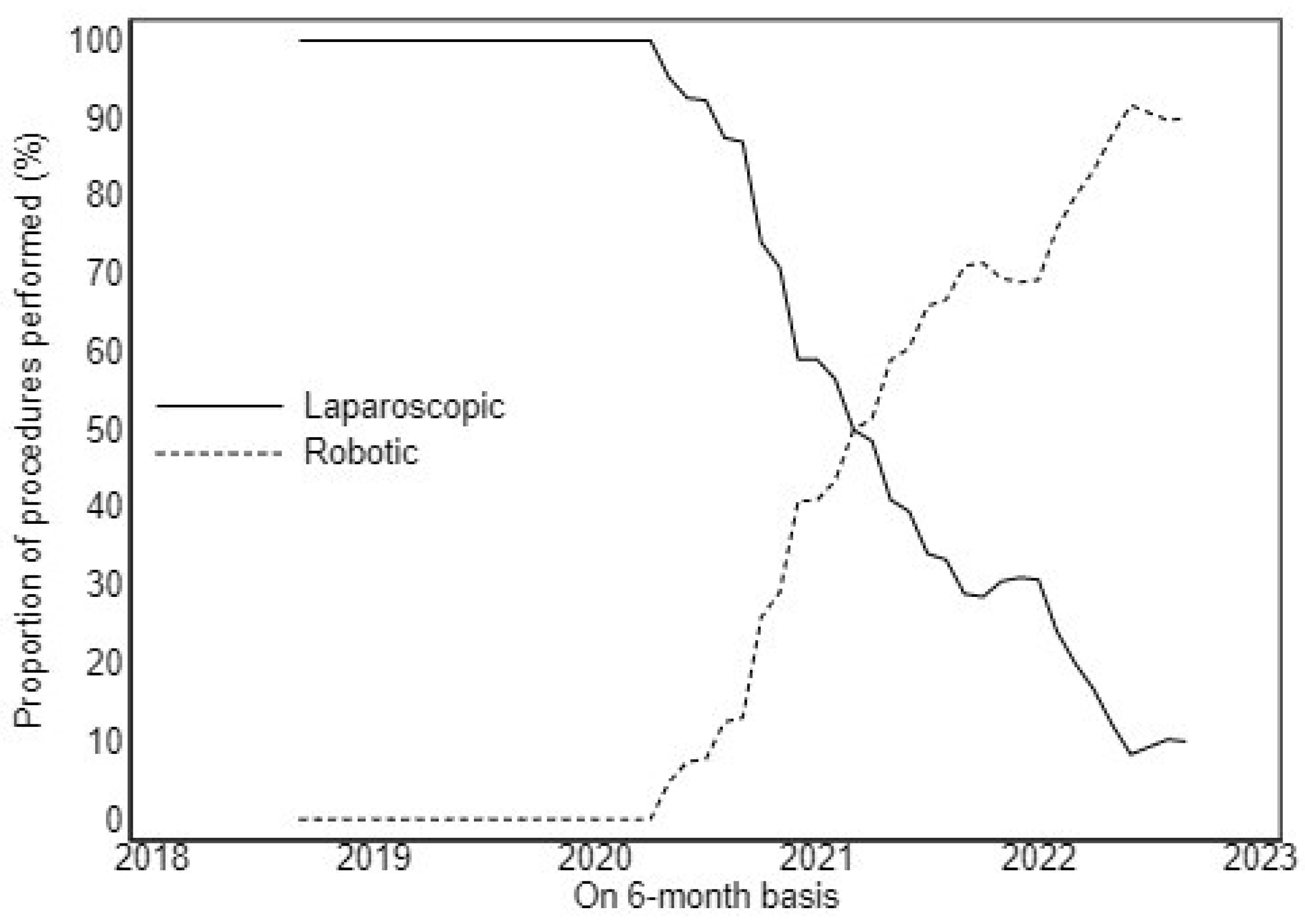
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’Rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. The International Position on Laparoscopic Liver Surgery: The Louisville Statement, 2008. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for Laparoscopic Liver Resection: A Report from the Second International Consensus Conference Held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [PubMed]
- Hilal, M.A.; Aldrighetti, Ã.L.; Dagher, I.; Aroori, S.; Belli, Ã.Ã.G.; Besselink, M.; Briceno, J.; Gayet, B.; Hondt, M.D.; Lesurtel, M.; et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef]
- Giulianotti, P.C. Robotics in General Surgery. Arch. Surg. 2003, 138, 777. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wakabayashi, G.; Kim, H.-J.; Choi, G.-H.; Yiengpruksawan, A.; Fong, Y.; He, J.; Boggi, U.; Troisi, R.I.; Efanov, M.; et al. International Consensus Statement on Robotic Hepatectomy Surgery in 2018. World J. Gastroenterol. 2019, 25, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, T.; Jensen, M.D.; Hartwell, D.; Mosgaard, B.J.; Jørgensen, A.; Jensen, B.R. Robotic Surgery Is Less Physically Demanding Than Laparoscopic Surgery: Paired Cross Sectional Study. Ann. Surg. 2020, 271, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kingham, T.P.; Leung, U.; Kuk, D.; Gönen, M.; D’Angelica, M.I.; Allen, P.J.; Dematteo, R.P.; Laudone, V.P.; Jarnagin, W.R.; Fong, Y. Robotic Liver Resection: A Case-Matched Comparison. World J. Surg. 2016, 40, 1422–1428. [Google Scholar] [CrossRef]
- Choi, G.H.; Chong, J.U.; Han, D.H.; Choi, J.S.; Lee, W.J. Robotic Hepatectomy: The Korean Experience and Perspective. Hepatobil. Surg. Nutr. 2017, 6, 230–238. [Google Scholar] [CrossRef]
- Morel, P.; Jung, M.; Cornateanu, S.; Buehler, L.; Majno, P.; Toso, C.; Buchs, N.C.; Rubbia-Brandt, L.; Hagen, M.E. Robotic versus Open Liver Resections: A Case-Matched Comparison. Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13, e1800. [Google Scholar] [CrossRef]
- Sucandy, I.; Giovannetti, A.; Ross, S.; Rosemurgy, A. Institutional First 100 Case Experience and Outcomes of Robotic Hepatectomy for Liver Tumors. Am. Surg. 2020, 86, 200–207. [Google Scholar] [CrossRef]
- Guan, R.; Chen, Y.; Yang, K.; Ma, D.; Gong, X.; Shen, B.; Peng, C. Clinical Efficacy of Robot-Assisted versus Laparoscopic Liver Resection: A Meta Analysis. Asian J. Surg. 2019, 42, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Moris, D.; Vagios, S.; Merath, K.; Pawlik, T.M. Safety and Oncologic Outcomes of Robotic Liver Resections: A Systematic Review. J. Surg. Oncol. 2018, 117, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Ciria, R.; Berardi, G.; Alconchel, F.; Briceño, J.; Choi, G.H.; Wu, Y.M.; Sugioka, A.; Troisi, R.I.; Salloum, C.; Soubrane, O.; et al. The Impact of Robotics in Liver Surgery: A Worldwide Systematic Review and Short-Term Outcomes Meta-Analysis on 2,728 Cases. J. Hepatobil. Pancreat. Sci. 2020, 29, 181–197. [Google Scholar] [CrossRef]
- Lafaro, K.J.; Stewart, C.; Fong, A.; Fong, Y. Robotic Liver Resection. Surg. Clin. N. Am. 2020, 100, 265–281. [Google Scholar] [CrossRef]
- Feldbrügge, L.; Ortiz Galindo, S.A.; Frisch, O.; Benzing, C.; Krenzien, F.; Riddermann, A.; Kästner, A.; Nevermann, N.F.; Malinka, T.; Schöning, W.; et al. Safety and Feasibility of Robotic Liver Resection after Previous Abdominal Surgeries. Surg. Endosc. 2022, 36, 2842–2849. [Google Scholar] [CrossRef]
- Delvecchio, A.; Conticchio, M.; Riccelli, U.; Ferraro, V.; Ratti, F.; Gelli, M.; Anelli, F.M.; Laurent, A.; Vitali, G.C.; Magistri, P.; et al. Laparoscopic versus Open Liver Resection for Hepatocellular Carcinoma in Elderly Patients: A Propensity Score Matching Analysis. HPB 2022, 24, 933–941. [Google Scholar] [CrossRef]
- Dindo, D.; Clavien, P.A. What Is a Surgical Complication? World J. Surg. 2008, 32, 942–944. [Google Scholar] [CrossRef]
- Idrees, K.; Bartlett, D.L. Robotic Liver Surgery. Surg. Clin. N. Am. 2010, 90, 761–774. [Google Scholar] [CrossRef]
- Casciola, L.; Patriti, A.; Ceccarelli, G.; Bartoli, A.; Ceribelli, C.; Spaziani, A. Robot-Assisted Parenchymal-Sparing Liver Surgery Including Lesions Located in the Posterosuperior Segments. Surg. Endosc. 2011, 25, 3815–3824. [Google Scholar] [CrossRef] [PubMed]
- Kitisin, K.; Packiam, V.; Bartlett, D.L.; Tsung, A. A Current Update on the Evolution of Robotic Liver Surgery. Minerva Chir. 2011, 66, 281–293. [Google Scholar] [PubMed]
- Montalti, R.; Scuderi, V.; Patriti, A.; Vivarelli, M.; Troisi, R.I. Robotic versus Laparoscopic Resections of Posterosuperior Segments of the Liver: A Propensity Score-Matched Comparison. Surg. Endosc. 2016, 30, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Szold, A.; Bergamaschi, R.; Broeders, I.; Dankelman, J.; Forgione, A.; Langø, T.; Melzer, A.; Mintz, Y.; Morales-Conde, S.; Rhodes, M.; et al. European Association of Endoscopic Surgeons (EAES) Consensus Statement on the Use of Robotics in General Surgery. Surg. Endosc. 2015, 29, 253–288. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, D.; Gonzalez-Heredia, R.; Brown, M.; Bianco, F.M.; Tzvetanov, I.; Davis, M.; Kim, J.; Benedetti, E.; Giulianotti, P.C. Financial Impact of the Robotic Approach in Liver Surgery: A Comparative Study of Clinical Outcomes and Costs between the Robotic and Open Technique in a Single Institution. J. Laparoendosc. Adv. Surg. Tech. 2017, 27, 375–382. [Google Scholar] [CrossRef]
- Chen, P.D.; Wu, C.Y.; Hu, R.H.; Chen, C.N.; Yuan, R.H.; Liang, J.T.; Lai, H.S.; Wu, Y.M. Robotic Major Hepatectomy: Is There a Learning Curve? Surgery 2017, 161, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Gravetz, A.; Sucandy, I.; Wilfong, C.; Patel, N.; Spence, J.; Ross, S.; Rosemurgy, A. Single-Institution Early Experience and Learning Curve with Robotic Liver Resections. Am. Surg. 2019, 85, 115–119. [Google Scholar] [CrossRef]
- Heemskerk, J.; Van Gemert, W.G.; De Vries, J.; Greve, J.; Bouvy, N.D. Learning Curves of Robot-Assisted Laparoscopic Surgery Compared with Conventional Laparoscopic Surgery: An Experimental Study Evaluating Skill Acquisition of Robot-Assisted Laparoscopic Tasks Compared with Conventional Laparoscopic Tasks in Inexperienced. Surg. Laparosc. Endosc. Percutaneous Tech. 2007, 17, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Wu, T.; Lin, J.; Deng, L.; Jiang, J.; An, T. Robotic versus Laparoscopic Major Hepatectomy for Hepatocellular Carcinoma: Short-Term Outcomes from a Single Institution. BMC Surg. 2022, 22, 432. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.; De’Angelis, N.; Beghdadi, N.; Brunetti, F.; Manigrasso, M.; De Simone, G.; Servillo, G.; Vertaldi, S.; De Palma, G.D. Conversions Related to Adhesions in Abdominal Surgery. Robotic versus Laparoscopic Approach: A Multicentre Experience. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2186. [Google Scholar] [CrossRef] [PubMed]
- Quijano, Y.; Vicente, E.; Ielpo, B.; Duran, H.; Diaz, E.; Fabra, I.; Malave, L.; Ferri, V.; Plaza, C.; Lindemann, J.L.; et al. Hepatobilio-Pancreatic Robotic Surgery: Initial Experience from a Single Center Institute. J. Robot. Surg. 2017, 11, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.A.C.; Lobo-Filho, M.M.; Mattos, B.H.; Ardengh, A.O.; Makdissi, F.F. Robotic Liver Resection. Report of the First 50 Cases. Arq. Gastroenterol. 2021, 58, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Fruscione, M.; Pickens, R.; Baker, E.H.; Cochran, A.; Khan, A.; Ocuin, L.; Iannitti, D.A.; Vrochides, D.; Martinie, J.B. Robotic-Assisted versus Laparoscopic Major Liver Resection: Analysis of Outcomes from a Single Center. HPB 2019, 21, 906–911. [Google Scholar] [CrossRef]
- Quijano, Y.; Vicente, E.; Ielpo, B.; Duran, H.; Diaz, E.; Fabra, I.; Olivares, S.; Ferri, V.; Ortega, I.; Malavé, L.; et al. Robotic Liver Surgery: Early Experience from a Single Surgical Center. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Liu, Y.; Paruch, J.L.; Zhou, L.; Kmiecik, T.E.; Ko, C.Y.; Cohen, M.E. Development and Evaluation of the Universal ACS NSQIP Surgical Risk Calculator: A Decision Aid and Informed Consent Tool for Patients and Surgeons. J. Am. Coll. Surg. 2013, 217, 833–842.e3. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Cheung, Y.S.; Chong, C.C.N.; Wong, J.; Fong, A.K.W.; Lai, P.B.S. Laparoscopic and Robotic Hepatectomy: Experience from a Single Centre. ANZ J. Surg. 2016, 86, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Guadagni, S.; Pesi, B.; Furbetta, N.; Di Franco, G.; Palmeri, M.; Annecchiarico, M.; Eugeni, E.; Coratti, A.; Patriti, A.; et al. Outcomes of Robotic Liver Resections for Colorectal Liver Metastases. A Multi-Institutional Analysis of Minimally Invasive Ultrasound-Guided Robotic Surgery. Surg. Oncol. 2019, 28, 14–18. [Google Scholar] [CrossRef]


| All | Median (Range Interquartile) | ||
|---|---|---|---|
| N | n = 100 | ||
| Age | 100 | 66 ± 12 | 68 (58–75) |
| Age ≥ 65 y | 100 | 58 (58.0%) | |
| Male | 100 | 59 (59.0%) | |
| BMI | 100 | 26.7 ± 3.2 | 27.0 (24.0–28.0) |
| ASA_I-II | 100 | 53 (53.0%) | |
| ASA_III-IV | 100 | 47 (47.0%) | |
| Previous open abdominal surgery | 100 | 34 (34.0%) | |
| Previous laparoscopic abdominal surgery | 100 | 26 (26.0%) | |
| Smoking | 100 | 16 (16.0%) | |
| Diabetes | 100 | 19 (19.0%) | |
| Cardiovascular disease | 100 | 52 (52.0%) | |
| Pulmonary diseases | 100 | 12 (12.0%) | |
| ALD | 100 | 5 (5%) | |
| NASH | 100 | 15 (15%) | |
| HBV | 100 | 9 (9%) | |
| HCV | 100 | 17 (17%) | |
| Other | 100 | 2 (2%) | |
| Cirrhosis | 100 | 32 (32.0%) | |
| MELD Score | 100 | 6.6 ± 1.7 | |
| Child–Pugh A | 100 | 97 (97%) | |
| Child–Pugh B | 100 | 3 (3%) | |
| CHARLSON Comorbidity score | 99 | 6.4 ± 2.8 | 7.0 (5.0–8.0) |
| Preoperative albumine level | 76 | 3.86 ± 0.49 | 3.90 (3.50–4.20) |
| CA199_preop | 74 | 98 ± 470 | 12 (3–27) |
| AFP_preop | 75 | 119 ± 757 | 3 (2–6) |
| ACE_preop | 73 | 36 ± 254 | 2 (2–4) |
| Preoperative chemoterapy | 100 | 25 (25%) | |
| Number of lesions > 1 | 100 | 26 (26%) | |
| Liver tumor size (mm) | 96 | 36 ± 28 | 25 (18–45) |
| N | |
|---|---|
| Adenoma | 5 |
| Hepatic cyst | 4 |
| FNH | 1 |
| Hemangioma | 3 |
| Intrahepatic lithiasis | 1 |
| HCC | 35 |
| iCCA | 3 |
| ADK cholec | 8 |
| CRLM | 27 |
| NCRLM | 13 |
| N | |
|---|---|
| Formal Left Hepatectomy | 4 |
| Formal Right Hepatectomy | 5 |
| Anatomical Resection | 27 |
| Non-Anatomical Resection | 63 |
| Central Hepatectomy | 1 |
| All | Median (Range Interquartile) | ||
|---|---|---|---|
| N | n = 100 | ||
| Difficult Index Score | |||
| Low | 100 | 42 (42.0%) | |
| Intermediate | 100 | 17 (17.0%) | |
| High | 100 | 41 (41.0%) | |
| Simultaneous procedure | 100 | 28 (28.0%) | |
| Rehepatectomy | 100 | 4 (4.0%) | |
| Conversion rate | 100 | 0 (0.0%) | |
| Operative time (min) | 100 | 302 ± 107 | 298 (240–360) |
| Estimated blood loss (mL) | 100 | 225 ± 178 | 200 (100–275) |
| Blood transfusion | 100 | 1 (1.0%) | |
| Pedicule clamping | 100 | 73 (73.0%) | |
| Total time of clamping (min) | 100 | 34 ± 32 | 30 (0–48) |
| Drain | 100 | 99 (99.0%) | |
| Postoperative complications | 100 | 15 (15.0%) | |
| Biliary leakage | 100 | 1 (1.0%) | |
| Hemorrhage | 100 | 0 (0.0%) | |
| Ascitis | 100 | 5 (5.0%) | |
| Pulmonary infection | 100 | 6 (6.0%) | |
| Other infection | 100 | 3 (3.0%) | |
| Clavien Dindo 1 | 100 | 68 (68.0%) | |
| Clavien Dindo 2 | 100 | 8 (8.0%) | |
| Clavien Dindo 3 | 100 | 1 (1.0%) | |
| Reintervention | 100 | 1 (1.0%) | |
| ICU stay days | 100 | 0.08 ± 0.27 | |
| Length of hospital stay | 100 | 5.5 ± 2.6 | 5.0 (4.0–6.0) |
| Postoperative CT scan | 100 | 23 (23.0%) | |
| Readmission at 90 d | 100 | 5 (5.0%) | |
| Death | 100 | 1 (1.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conticchio, M.; Delvecchio, A.; Ferraro, V.; Stasi, M.; Casella, A.; Filippo, R.; Tedeschi, M.; Fiorentino, A.; Memeo, R. Robotic Liver Resection: Report of Institutional First 100 Cases. Surg. Tech. Dev. 2023, 12, 176-187. https://doi.org/10.3390/std12040017
Conticchio M, Delvecchio A, Ferraro V, Stasi M, Casella A, Filippo R, Tedeschi M, Fiorentino A, Memeo R. Robotic Liver Resection: Report of Institutional First 100 Cases. Surgical Techniques Development. 2023; 12(4):176-187. https://doi.org/10.3390/std12040017
Chicago/Turabian StyleConticchio, Maria, Antonella Delvecchio, Valentina Ferraro, Matteo Stasi, Annachiara Casella, Rosalinda Filippo, Michele Tedeschi, Alba Fiorentino, and Riccardo Memeo. 2023. "Robotic Liver Resection: Report of Institutional First 100 Cases" Surgical Techniques Development 12, no. 4: 176-187. https://doi.org/10.3390/std12040017
APA StyleConticchio, M., Delvecchio, A., Ferraro, V., Stasi, M., Casella, A., Filippo, R., Tedeschi, M., Fiorentino, A., & Memeo, R. (2023). Robotic Liver Resection: Report of Institutional First 100 Cases. Surgical Techniques Development, 12(4), 176-187. https://doi.org/10.3390/std12040017







