1. Introduction
The insertion of a functional and durable peritoneal dialysis catheter (PDC) is critical for successful peritoneal dialysis (PD). Many techniques for PDC insertion are available, including laparoscopic, open surgical, fluoroscopic-guided, and blind Seldinger techniques. The method of choice depends on the institutional resources and expertise [
1]. Ultrasound (US)-guided PDC insertion has been previously described, especially in the blind Seldinger technique, where it is adopted to avoid major vessel and bowel injury and to ensure that the tip of the PDC is adequately placed in the deep pelvis [
2].
The minimally invasive laparoscopic technique for PDC insertion is a well-known and established technique worldwide and has been implemented in our institution for the past 15 years [
3]. However, despite good results and very low complication rates, we have observed a high frequency of pericatheter dialysate fluid leaks into the abdominal wall, around the PDC and from the skin exit site, in our case series of 40 patients who had laparoscopic PDC insertions. Pericatheter dialysate fluid leak is an unwanted complication that can increase the risk of infective complications and PD failure. Therefore, properly positioning the PDC, especially the internal cuff, which should lie inside the rectus muscle sheath, is paramount for minimizing a potential dialysate fluid leak [
4]. Accordingly, we have upgraded the previous technique with intraoperative US evaluation of the abdominal wall with measurement of the thickest part of the rectus abdominis muscle while localizing the inferior epigastric artery and its major branches. The modified technique and its advantages are briefly described in this paper.
2. Case Presentation
In all candidates for peritoneal dialysis (PD), a laparoscopic insertion of PDC under general anesthesia has been performed in our institution for the past 15 years. Recently, we implemented a modified technique with intraoperative US evaluation of the abdominal wall on the side of PDC insertion. The abdominal surgeon performs all PDC insertions under general anesthesia, while the nephrologist joins the surgery to perform an US of the abdominal wall. The side of PDC is agreed upon with patients preoperatively after obtaining informed consent. The patient is placed supine, and a single preoperative antibiotic prophylaxis with 1 g to 2 g cefazolin is given 30 min before the skin incision. If a penicillin allergy is known, patients get 500 mg of vancomycin. The sterile operative field is prepared per standard protocol. Thereafter, the position of the internal cuff and a skin exit site are marked. The coiled Tenckhoff catheters with two dacron cuffs and a special trocar are used for rectus sheath tunneling. When the procedure is commenced, an US evaluation of the abdominal wall is performed ipsilaterally to the PDS insertion into the abdominal cavity. (
Figure 1). The rectus abdominis muscle width is measured with the US (
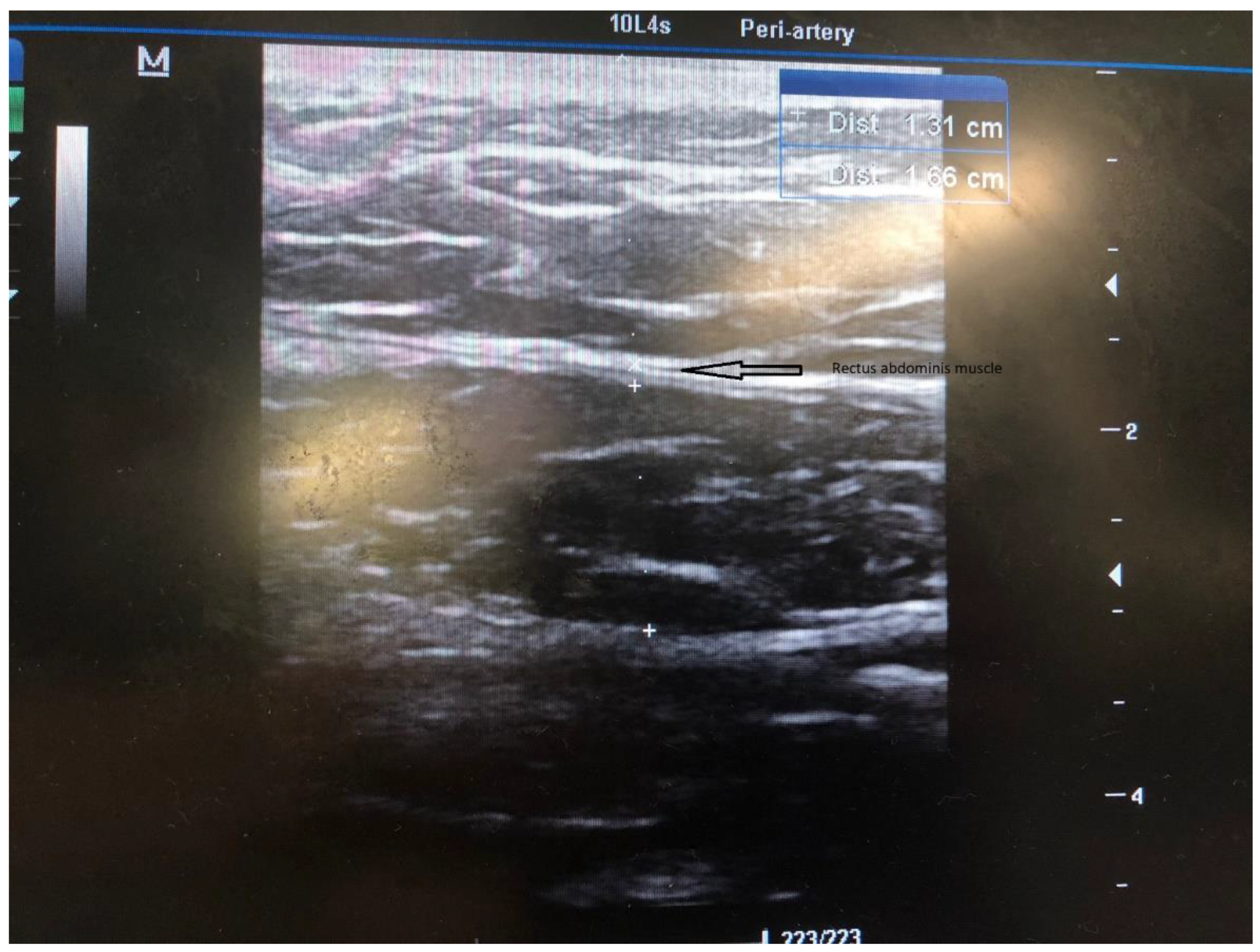
Figure 2), and the course of the inferior epigastric artery is determined with colored US Doppler (
Figure 3). On the abdominal wall, the thickest part of the rectus abdominis muscle and the course of the inferior epigastric artery are both marked with a pencil (
Figure 4). After that, the position of the internal cuff is determined 2–3 cm laterally from the umbilicus, where the rectus muscle is sufficiently thick. The positions of the external cuff and the skin exit site are also marked on the skin before insertion. After the US evaluation of the abdominal wall is completed, the laparoscopic insertion of PDC is performed. A standard laparoscopy is performed with one 5 mm trocar above the umbilicus and a 5 mm 30-degree camera. The second 5 mm trocar is inserted laterally as a working trocar for a laparoscopic grasper. The 5 mm skin incision is initially performed above the umbilicus, and a Veress needle is blindly introduced into the abdominal cavity. Aspiration and a water drop test is performed to ensure that the Veress needle is properly inside the peritoneal cavity. Pneumoperitoneum is created with CO
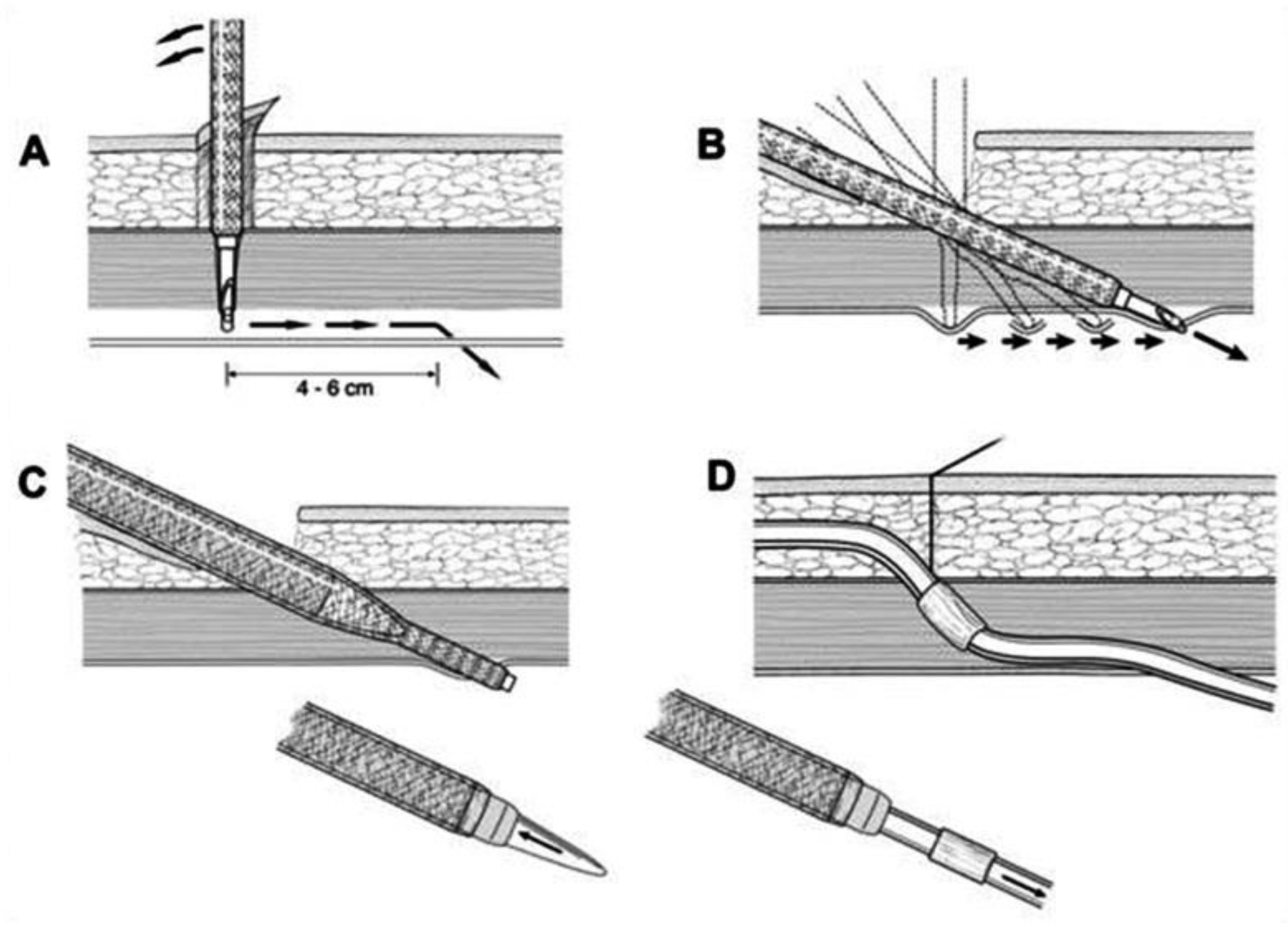
2, and the intraabdominal pressure is maintained around 12 mm Hg, as in standard laparoscopy. A 5 mm trocar is blindly introduced into the abdominal cavity at this site. Although all patients have preoperative abdominal US performed, we further perform diagnostic laparoscopy with a 5 mm 30-degree camera to exclude any other associated intraabdominal pathology. Contralateral to the PDC insertion, another 5 mm trocar is inserted under laparoscopic vision and a camera is moved to that trocar so that the entry site of the catheter into the peritoneal cavity can be visualized during insertion. The patient is tilted to a slight Trendelenburg position, thus allowing a better exposure of the deep pelvis, which is the point for inserting the PDC tip. The Trendelenburg position enables the small bowel loops to retreat from the pelvis so that the pelvis can be further inspected for possible adhesion. The advantage of laparoscopy is also to enable advanced laparoscopic procedures, such as adhesiolysis if needed. Thereafter, the 1 cm skin incision is performed ipsilateral to the side of PDC placement, and a special trocar is used for rectus sheath tunneling. Because of the preoperative US evaluation of the abdominal wall, we are aware of the inferior epigastric artery course, so we can accommodate the rectus sheath tunneling course so as to avoid vessel injury and bleeding. Rectus sheath tunneling is performed with a special trocar, through which the PDC is then inserted into the abdominal cavity. The trocar is introduced under a 45-degree angle through a small skin incision into the subcutaneous tissue and is then blindly forwarded through the abdominal wall (
Figure 5 and
Figure 6). The peritoneum is penetrated under direct laparoscopic vision to avoid any bowel or large vessel injury. The PDC is introduced through the trocar into the abdominal cavity, and the trocar is removed. With an atraumatic laparoscopic grasper, the coiled tip of the PDC is placed into the deep pelvis and the inner cuff is placed preperitoneally under direct laparoscopic supervision. The PDC is exteriorized laterally, and the outer cuff is placed in the subcutaneous tissue. The distance between the outer cuff and the skin exit site should be 2–4 cm. At the end of the laparoscopy, the proper position of the PDC tip and potential bleeding from the peritoneum at the trocar and PDC breakthrough sites are rechecked, respectively. All trocars and working instruments are removed from the abdominal cavity, and the pneumoperitoneum is released. The PDC is flushed with 50 mL of normal sterile saline to ensure the fluid flows freely in and out of the abdominal cavity. The fascia at trocar sites is sutured, and skin incisions are sutured with 4/0 absorbable sutures. The latter are infiltrated with a local anesthetic. When the surgical part of the PDC insertion is completed, an US is performed again to check for a proper position of the PDC tip and inner cuff. The outer end of the PDC is connected to the titanium adapter and transfer set. The complete outer part of the PDC is covered with gauze and transparent dressing. Following the procedure, the patient is admitted to the nephrology department, where a small volume flushing (200–500 mL) is started the next day. According to our institutional protocol, after laparoscopic insertion of the PDC, all patients have to wait 4–6 weeks before a full PD is commenced to achieve full healing of both the skin and abdominal wall wounds at trocar sites, thereby preventing (or minimizing) the possible leak. The patient is advised to avoid heavy lifting and strenuous physical activity for 4–6 weeks after the procedure.
3. Discussion
Peritoneal dialysis is an established treatment method for end-stage renal failure. It is used in motivated and reliable patients whose renal function has already or will shortly deteriorate to the point that dialysis is required [
1,
2,
3]. There are many techniques of PDC placement, such as open surgical, laparoscopic, peritoneoscopic, and blind Seldinger techniques. The laparoscopic technique is commonly used by surgeons, while other techniques are also used by nephrologists dealing with PDC insertions [
1,
2,
3]. US-guided placement of PDC has already been described in a blind Seldinger technique for PDC placement to avoid perioperative complications such as bowel perforation and bleeding [
2]. It is used mainly by nephrologists dealing with PDC insertions. In our institution, PDC catheters are inserted laparoscopically by one surgeon skilled in laparoscopy.
The laparoscopic technique of PDC insertion is a well-known and established technique for PDC placement. The intraabdominal placement of the PDC is performed under the direct vision of a laparoscope, which helps to avoid injury to the bowel and to place the PDC tip correctly in the deep pelvis. A critically important component of PDC placement is a proper rectus sheath tunneling with a special trocar, which is performed blindly, without guidance on the location of the major vessels and where the rectus muscle is sufficiently thick for the proper placement of the internal cuff that should lie within the rectus sheath preperitoneally [
5]. The other possible complication of rectus sheath tunneling is an injury of the inferior epigastric artery with major bleeding, which can require a more aggressive approach, such as laparotomy, to arrest bleeding. A crucial step in PDC placement is the proper position of the internal and external cuff and proper exteriorization of the PDC. The internal cuff should not slip into the abdominal cavity, which can be seen and repaired during laparoscopy. However, during laparoscopy, we cannot see if the internal cuff is placed properly within the rectus sheath. In patients with a very thin abdominal wall and thin rectus muscle, the internal cuff can slip out of the rectus sheath into the subcutaneous tissue, which can be seen with US and not with laparoscopy. The proper position of the internal cuff is essential for the proper incorporation of the PDC into the abdominal wall, which reduces the possibility of a pericatheter dialysate fluid leak and potential PD failure [
6]. The advantage of inserting the catheter through the thickest part of the muscle is that the internal cuff has more place to be incorporated within the rectus muscle. If the patient has a thin abdominal wall with a weak rectus muscle, the internal cuff is pulled through the muscle and incorporated preperitoneally to provide the optimal position and prevent a possible leak of dialysate fluid.
In our experience, US guidance is of great benefit for correctly positioning the PDC into the abdominal wall. It allows us to determine the thickest part of the rectus abdominis muscle and the ideal site of the internal cuff position within the rectus sheath. In addition, the US-Doppler can help to identify the course of the inferior epigastric artery and its major branches and thus helps avoid major bleeding during surgery. After the PDC insertion, the US helps us to check the proper position of the internal cuff and to correct the position if necessary [
7]. Although the laparoscopic technique is very effective and safe, it does not preclude complications during blind rectus sheath tunnelling. Hence, we opted to modify our previous technique to ensure the safest and most effective approach. Moreover, we noticed increased pericatheter dialysate fluid leaks in our last 40 patients. All these patients were evaluated with US, and in a few of them, we observed the internal cuff either displaced into the subcutaneous tissue or placed in a suboptimal place within a very thin rectus muscle. Besides the technical failure, other factors predispose to a pericatheter leak, such as a thin abdominal wall, bad tissue healing, and other patient conditions. Hence, we decided to optimize the technical aspect to perform the most optimal PDC insertion. We believe that the afore-described technique presents a step forward, and we see a great benefit of it in providing the most optimal PDC insertion while reducing the technical failure of PD. We believe that combining preoperative and intraoperative US evaluation of the abdominal wall with concurrent use of laparoscopy is the safest and most effective technique of PDC insertion. However, so far, we have performed the modified US-guided technique in only a few of our patients, and we do not have data on long-term results. Due to the small number of patients, at the moment, we cannot objectively evaluate the results and compare both techniques.
While US is very effective as a diagnostic imaging modality for assessing complications in peritoneal dialysis, it is also very applicable as an intraoperative imaging tool during PDC placement. The US examination can also be applied for preoperative assessment, during the peritoneal catheter placement, for detecting and monitoring infection, and for evaluating the catheter malfunction. Despite being a cost- and time-saving technique and a bedside procedure, US remains an underrated clinical tool in the field of peritoneal dialysis [
8]. For sonographic assessment of the PDC, a linear (high frequency) transducer is preferred as it provides adequate visualization of the superficial structures including the catheter. It is possible to trace the catheter all the way to its disappearance or downward deep into the rectus muscle in most of the individuals. This may prove tricky in obese patients in whom PDC can course a little deeper. In those patients, the curvilinear (abdominal) probe can be used to visualize the PDC entry into the rectus sheath. A vascular preset is preferred to prevent noise from superficial structures and movement with respiration [
8,
9]. Contrast enhanced ultrasound (CEUS) is an upgrade to standard US, which is very helpful in evaluating PDC malfunction, peritoneal-pleural communication, leakage, and herniation, and in particular, it facilitates dynamic functional imaging of the catheter and its complications. The use of CEUS in peritoneal dialysis is simple, repeatable, safe, radiation-free, and appears to be less time-consuming and more cost-effective than other radiological imaging techniques such as peritoneography, computed tomography, magnetic resonance, and peritoneal scintigraphy [
10].
US can be a useful tool in those patients that are more prone to bleeding, such as patients on anticoagulant therapy and patients with haemathological diseases or any other bleeding disorders. It can be used as a bedside tool or at the end of the surgery for evaluation of possible bleeding within the abdominal wall, which can occur during rectus sheath tunneling [
8]. Rectus sheath tunneling is an important step in PDC placement, which is performed blindly with a trocar [
5]. Even though the surgeon is aware of the inferior epigastric artery course, the bleeding can occur from small vessels, especially in patients with any kind of bleeding disorder. US is also useful for evaluating other complications, especially PD-related infectious complications. Despite great efforts in preventing PD-related infectious complications, approximately one third of PD failures are caused by them [
9]. Hence, prompt exit site or tunnel infection diagnosis would allow fast and the most appropriate treatment, thereby decreasing the potential complications and reducing the PD failure rate [
9].
In conclusion, we can say that US could be used more frequently as a helping tool in PDC placement to avoid unnecessary complications and to provide the most optimal placement of the PDC.