Upper-Abdominal Cytoreduction for Advanced Ovarian Cancer—Therapeutic Rationale, Surgical Anatomy and Techniques of Cytoreduction
Abstract
1. Introduction
2. Benefit of Upper-Abdominal Cytoreduction
3. Pattern of Peritoneal Dissemination and Involvement of the Upper Abdomen
3.1. Lymph Node Involvement
3.2. Parenchymal Metastases
4. Surgical Anatomy of the Upper Abdomen
4.1. Peritoneal Ligaments and Spaces
4.2. Anatomical Boundaries from the Surgical Viewpoint
4.3. Regional Nodes
5. Preoperative Imaging
6. Technical Aspects of Upper-Abdominal Cytoreduction
6.1. Energy Devices
6.2. Right Subphrenic Peritonectomy
6.3. Glissonectomy
6.4. Left Subphrenic Peritonectomy and Central Diaphragmatic Peritonectomy
6.5. Lesser Omentectomy and Hepatoduodenal Ligament Clearance
6.6. Foramen of Winslow and the Posterior Layer of the Hepatoduodenal Ligament
6.7. Superior Recess of the Lesser Sac
6.8. Inferior Recess of the Lesser Sac
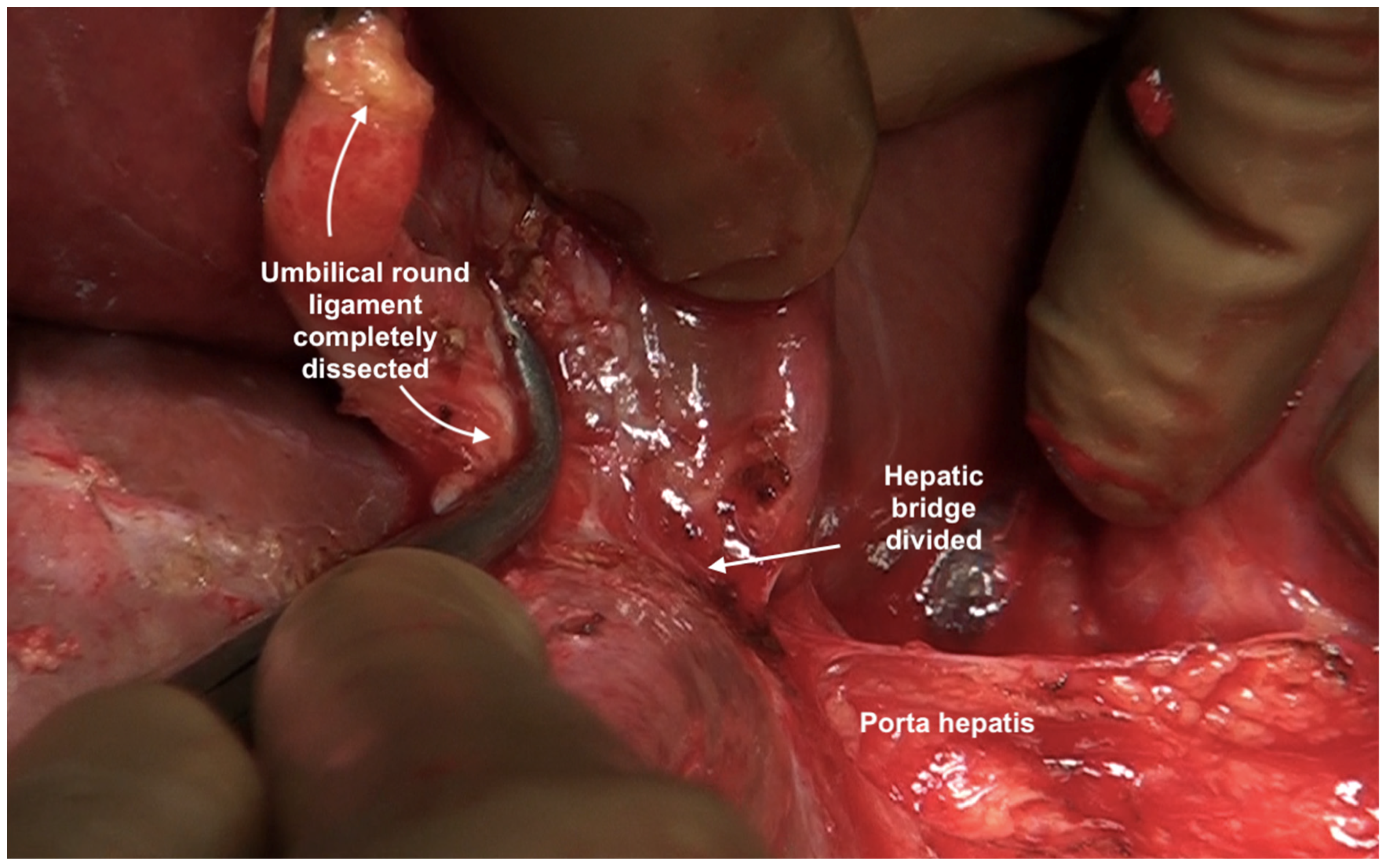
6.9. Umbilical Round Ligament
6.10. The Peritoneum Overlying the Pancreas
6.11. Lymphadenectomy
6.12. Topical Hemostatic Agents
6.13. Role of Minimally Invasive Surgery
- Even when there is limited disease, it is always accompanied by disease at other sites making the procedure technically challenging and time consuming if the peritonectomies are performed as described in the manuscript. We support our stand with the patient selection criteria for the LANCE trial in which patients with diaphragm, liver and splenic involvement are excluded from the trial [80].
- Though some papers show that for limited disease, MIS produces a survival compared to open surgery, the comparison is made between patients with limited disease undergoing MIS and the whole cohort of ovarian cancer patients undergoing open surgery which in our opinion is not a valid comparison [81]. Moreover, a study from the PSOGI registry showed a higher incidence of recurrence following MIS CRS for high-grade malignancies such as colorectal and ovarian cancer [82].
- For recurrent ovarian cancer, there could be two scenarios-either the diaphragm was previously stripped and there is a recurrence in the same region; in this scenario minimally invasive surgery is dangerous as there could be dense adhesions and without the tactile sensation that is available in open surgery, adhesionolysis should not be performed. In the second scenario, if the diaphragm was not stripped, we would perform a complete stripping in that region as described above.
- 4.
- There is a risk of missing small tumor nodules and some regions such as region 2 which is described above cannot be explored properly on MIS.
7. Morbidity of Upper-Abdominal Cytoreduction and Its Prevention
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar] [PubMed]
- Healy, J.C.; Reznek, R.H. The peritoneum, mesenteries and omenta: Normal anatomy and pathological processes. Eur. Radiol. 1998, 8, 886–900. [Google Scholar] [CrossRef] [PubMed]
- Pathiraja, P.N.; Garruto-Campanile, R.; Tozzi, R. Diaphragmatic peritonectomy versus full thickness diaphragmatic resection and pleurectomy during cytoreduction in patients with ovarian cancer. Int. J. Surg. Oncol. 2013, 2013, 876150. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Poynor, E.A.; Aghajanian, C.; Jarnagin, W.R.; Chi, D.S.; Barakat, R.R.; DeMatteo, R.P.; et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol. Oncol. 2006, 103, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Eng, O.S.; Raoof, M.; Blakely, A.M.; Yu, X.; Lee, S.J.; Han, E.S.; Wakabayashi, M.T.; Yuh, B.; Lee, B.; Dellinger, T.H. A collaborative surgical approach to upper and lower abdominal cytoreductive surgery in ovarian cancer. J. Surg. Oncol. 2018, 118, 121–126. [Google Scholar] [CrossRef]
- Meyers, M.A. Distribution of intra-abdominal malignant seeding: Dependency on dynamics of flow of ascitic fluid. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1973, 119, 198–206. [Google Scholar] [CrossRef]
- Pannu, H.K.; Bristow, R.E.; Montz, F.J.; Fishman, E.K. Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics 2003, 23, 687–701. [Google Scholar] [CrossRef]
- Martín-Cameán, M.; Delgado-Sánchez, E.; Piñera, A.; Diestro, M.D.; De Santiago, J.; Zapardiel, I. The role of surgery in advanced epithelial ovarian cancer. Ecancermedicalscience 2016, 10, 666. [Google Scholar] [CrossRef]
- Bianchi, F.; Camargo, A.; Habich, D.; Castano, R. The fundamental role of the exploration of the upper abdomen in ovarian cancer surgery. Obstet. Gynecol. Int. J. 2021, 12, 337–342. [Google Scholar] [CrossRef]
- Bhatt, A.; Parikh, L.; Mishra, S.; Glehen, O. Epithelial Serous Ovarian Cancer: Patterns of Peritoneal Dissemination and their Clinical Implications. In Pathology of Peritoneal Metastases; Glehen, O., Bhatt, A., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol. Oncol. 2009, 114, 26–31. [Google Scholar] [CrossRef]
- Chang, S.J.; Bristow, R.E.; Chi, D.S.; Cilby, W.A. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J. Gynecol. Oncol. 2015, 26, 336–342. [Google Scholar] [CrossRef]
- Harter, P.; Muallem, Z.M.; Buhrmann, C.; Lorenz, D.; Kaub, C.; Hils, R.; Kommoss, S.; Heitz, F.; Traut, A.; du Bois, A. Impact of a structured quality management program on surgical outcome in primary advanced ovarian cancer. Gynecol. Oncol. 2011, 121, 615–619. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, R.; Yin, S.; You, C.; Liu, D.; Cheng, X.; Tang, J.; Zang, R. Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: An observational study. BMC Cancer 2015, 15, 583. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, C.R.; Wilson, T.O.; Podratz, K.C.; Cilby, W.A. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006, 107, 77–85. [Google Scholar] [CrossRef]
- Colombo, P.E.; Mourregot, A.; Fabbro, M.; Gutowski, M.; Saint-Aubert, B.; Quenet, F.; Gourgou, S.; Rouanet, P. Aggressive surgical strategies in advanced ovarian cancer: A monocentric study of 203 stage IIIC and IV patients. Eur. J. Surg. Oncol. 2009, 35, 135–143. [Google Scholar] [CrossRef]
- Chang, S.J.; Bristow, R.E.; Ryu, H.S. Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann. Surg. Oncol. 2012, 19, 4059–4067. [Google Scholar] [CrossRef]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.W.; Park, S.Y.; Kim, B.G.; Nam, J.H.; et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef]
- Zang, R.Y.; Harter, P.; Chi, D.S.; Sehouli, J.; Jiang, R.; Tropé, C.G.; Ayhan, A.; Cormio, G.; Xing, Y.; Wollschlaeger, K.M.; et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br. J. Cancer 2011, 105, 890–896. [Google Scholar] [CrossRef]
- Classe, J.M.; Glehen, O.; Decullier, E.; Bereder, J.M.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for First Relapse of Ovarian Cancer. Anticancer Res. 2015, 35, 4997–5005. [Google Scholar] [PubMed]
- Bhatt, A.; Bakrin, N.; Gertych, W.; Kammar, P.; Parikh, L.; Sheth, S.; Shaikh, S.; Devouassoux-Shisheboran, M.; Glehen, O. Extent and distribution of peritoneal disease in patients undergoing cytoreductive surgery for first platinum sensitive recurrence in ovarian cancer and its potential therapeutic implications. Eur. J. Surg. Oncol. 2020, 46, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Bacalbasa, N.; Dima, S.; Balescu, I.; David, L.; Brasoveanu, V.; Popescu, I. Results of Primary Cytoreductive Surgery in Advanced-stage Epithelial Ovarian Cancer: A Single-center Experience. Anticancer Res. 2015, 35, 4099–4104. [Google Scholar] [PubMed]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2018, 143, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Kawamura, T.; Bandou, E.; Tsukiyama, G.; Endou, Y.; Miura, M. The natural history of free cancer cells in the peritoneal cavity. In Advances in Peritoneal Surface Oncology; Gonzalez-Moreno, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 11–23. [Google Scholar]
- Kusamura, S.; Baratti, D.; Zaffaroni, N.; Villa, R.; Laterza, B.; Balestra, M.R.; Deraco, M. Pathophysiology and biology of peritoneal carcinomatosis. World J. Gastrointest. Oncol. 2010, 2, 12–18. [Google Scholar] [CrossRef]
- Sehouli, J.; Senyuva, F.; Fotopoulou, C.; Neumann, U.; Denkert, C.; Werner, L.; Gülten, O.O. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J. Surg. Oncol. 2009, 99, 424–427. [Google Scholar] [CrossRef]
- Bhatt, A.; Kammar, P.; Rousset, P.; Sinukumar, S.; Mehta, S.; Parkih, L.; Goswami, G.; Sheikh, S.; Kepenekian, V.; Bakrin, N.; et al. Greater-omentum lesion-score (GOLS) as a predictor of residual disease in different regions of the peritoneal cavity in patients undergoing interval cytoreductive surgery for advanced ovarian cancer and its potential clinical utility. Eur. J. Surg. Oncol. 2021, 47, 2925–2932. [Google Scholar] [CrossRef]
- Bhatt, A.; Yonemura, Y.; Mehta, S.; Benzerdjeb, N.; Kammar, P.; Parikh, L.; Shah, M.Y.; Shaikh, S.; Prabhu, A.; Mishra, S.; et al. Target region resection in patients undergoing cytoreductive surgery for peritoneal metastases-is it necessary in absence of visible disease? Eur. J. Surg. Oncol. 2020, 46, 582–589. [Google Scholar] [CrossRef]
- Bhatt, A.; Yonemura, Y.; Benzerdjeb, N.; Mehta, S.; Mishra, S.; Parikh, L.; Kammar, P.; Shah, M.Y.; Prabhu, A.; Shaikh, S.; et al. Pathological assessment of cytoreductive surgery specimens and its unexplored prognostic potential—A prospective multi-centric study. Eur. J. Surg. Oncol. 2019, 45, 2398. [Google Scholar] [CrossRef]
- Silverman, P.M. The subperitoneal space: Mechanisms of tumour spread in the peritoneal cavity, mesentery, and omentum. Cancer Imaging. 2003, 4, 25–29. [Google Scholar] [CrossRef][Green Version]
- Fritz, D.L.; Waag, D.M. Transdiaphragmatic lymphatic transport of intraperitoneally administered marker in hamsters. Lab. Anim. Sci. 1999, 49, 522–529. [Google Scholar] [PubMed]
- Abernethy, N.J.; Chin, W.; Hay, J.B.; Rodela, H.; Oreopoulos, D.; Johnston, M.G. Lymphatic drainage of the peritoneal cavity in sheep. Am. J. Physiol. 1991, 260, F353–F358. [Google Scholar] [CrossRef] [PubMed]
- Parungo, C.P.; Soybel, D.I.; Colson, Y.L.; Kim, S.W.; Ohnishi, S.; DeGrand, A.M.; Laurence, R.G.; Soltesz, E.G.; Chen, F.Y.; Cohn, L.H.; et al. Lymphatic drainage of the peritoneal space: A pattern dependent on bowel lymphatics. Ann. Surg. Oncol. 2007, 14, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Luna-Abanto, J.; García Ruiz, L.; Laura Martinez, J.; Álvarez Larraondo, M.; Villoslada Terrones, V. Liver Resection as Part of Cytoreductive Surgery for Ovarian Cancer. J. Gynecol. Surg. 2020, 36, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.J.; Long, K.C.; Feffer, J.B.; Leitao, M.M., Jr.; Abu-Rustum, N.R.; Barakat, R.R.; Chi, D.S.; Gardner, G.J. Parenchymal splenic metastasis is an independent negative predictor of overall survival in advanced ovarian, fallopian tube, and primary peritoneal cancer. Gynecol. Oncol. 2013, 128, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Madambath, J.G.; Somani, P.; Pathak, A.; Rameshbabu, C.S.; Bansal, R.; Ramasamy, K.; Patil, A. Endoscopic ultrasound of peritoneal spaces. Endosc. Ultrasound. 2017, 6, 90–102. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In Peritoneal Carcinomatosis: Principles of Management; Sugarbaker, P.H., Ed.; Kluwer: Boston, MA, USA, 1996; pp. 359–374. [Google Scholar]
- Meyers, M.A.; Charnsangavej, C.; Oliphant, M. Meyers’ Dynamic Radiology of the Abdomen, 6th ed.; Springer: New York, NY, USA, 2011; pp. 23–34. [Google Scholar]
- Kim, S.; Kim, T.U.; Lee, J.W.; Lee, T.H.; Lee, S.H.; Jeon, T.Y.; Kim, K.H. The perihepatic space: Comprehensive anatomy and CT features of pathologic conditions. Radiographics 2007, 27, 129–143. [Google Scholar] [CrossRef]
- Villeneuve, L.; Thivolet, A.; Bakrin, N.; Mohamed, F.; Isaac, S.; Valette, P.J.; Glehen, O.; Rousset, P.; BIG-RENAPE and RENAPE Working Groups. A new internet tool to report peritoneal malignancy extent. PeRitOneal MalIgnancy Stage Evaluation (PROMISE) application. Eur. J. Surg. Oncol. 2016, 42, 877–882. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Bhatt, A.; Mehta, S. Cytoreductive Surgery for Peritoneal Metastases: Principles and Techniques. In Management of Peritoneal Metastases-Cytoreductive Surgery, HIPEC and Beyond; Bhatt, A., Ed.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Lee, I.O.; Lee, J.Y.; Kim, H.J.; Nam, E.J.; Kim, S.; Kim, S.W.; Lee, C.Y.; Kang, W.J.; Kim, Y.T. Prognostic significance of supradiaphragmatic lymph node metastasis detected by 18F-FDG PET/CT in advanced epithelial ovarian cancer. BMC Cancer 2018, 18, 1165. [Google Scholar] [CrossRef]
- Buza, N. Staging. PathologyOutlines.com website. Available online: https://www.pathologyoutlines.com/topic/ovarytumorstage.html (accessed on 24 December 2021).
- Suppiah, S. The Past, Present and Future of Diagnostic Imaging in Ovarian Cancer. In Ovarian Cancer–From Pathogenesis to Treatment, Omer Devaja and Andreas Papadopoulos; IntechOpen: London, UK, 24 October; Available online: https://www.intechopen.com/chapters/59552 (accessed on 29 September 2022). [CrossRef][Green Version]
- Verri, D. Pre-operative evaluation in advanced ovarian cancer: Is ultrasound ready to replace computed tomography? Int. J. Gynecol. Cancer 2019, 29, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Goswami, G.; Kammar, P.; Mangal, R.; Shaikh, S.; Patel, M.D.; Bhatt, A. Accuracy of CT Scan in Predicting the Surgical PCI in Patients Undergoing Cytoreductive Surgery with/without HIPEC-a Prospective Single Institution Study. Indian J. Surg. Oncol. 2019, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.A.; Khader, L.; Cirigliano, A.; Cioffi Squitieri, N.; Guerrini, S.; Forzoni, B.; Marrelli, D.; Roviello, F.; Mazzei, F.G.; Volterrani, L. Accuracy of MDCT in the preoperative definition of Peritoneal Cancer Index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Abdom. Imaging. 2013, 38, 1422–1430. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M.; Lucero, J. Comparison of MRI and CT for predicating the peritoneal cancer index (PCI) in patients being considered for cytoreductive surgical procedures. Ann. Surg. Oncol. 2015, 22, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Dohan, A.; Hoeffel, C.; Soyer, P.; Jannot, A.S.; Valette, P.J.; Thivolet, A.; Passot, G.; Glehen, O.; Rousset, P. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br. J. Surg. 2017, 104, 1244–1249. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M.; Rousset, P. Peritoneal MRI in patients undergoing cytoreductive surgery and HIPEC: History, clinical applications, and implementation. Eur. J. Surg. Oncol. 2021, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Llueca, A.; Serra, A.; Rivadulla, I.; Gomez, L.; Escrig, J.; MUAPOS working group (Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery). Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index. World J. Surg. Oncol. 2018, 16, 37. [Google Scholar] [CrossRef]
- Mercier, F.; Mohamed, F.; Cazauran, J.B.; Kepenekian, V.; Vaudoyer, D.; Cotte, E.; Glehen, O.; Passot, G. An update of peritonectomy procedures used in cytoreductive surgery for peritoneal malignancy. Int. J. Hyperthermia. 2019, 36, 744–752. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Dagbert, F.; Passot, G.; Glehen, O.; Bakrin, N. Glisson capsulectomy for extensive superficial liver involvement in peritoneal carcinomatosis (with video). J. Visc. Surg. 2015, 152, 332–333. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Dissection by electrocautery with a ball tip. J. Surg. Oncol. 1994, 56, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Boerner, T.; Piso, P. Cytoreductive Surgery for Peritoneal Carcinomatosis from Gastric Cancer: Technical Details. J. Clin. Med. 2021, 10, 5263. [Google Scholar] [CrossRef] [PubMed]
- Vizzielli, G.; Conte, C.; Romano, M.; Fagotti, A.; Costantini, B.; Lodoli, C.; Gueli Alletti, S.; Gaballah, K.; Pacelli, F.; Ercoli, A.; et al. Clinical Impact of a Surgical Energy Device in Advanced Ovarian Cancer Surgery Including Bowel Resection. In Vivo 2018, 32, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Seror, J.; Bats, A.S.; Habchi, H.; Lécuru, F. Optimal surgical cytoreduction of the upper abdomen and the diaphragm for advanced ovarian cancer using PlasmaJet™ energy. Gynecol. Oncol. 2016, 140, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Fournier, I.; Christodoulou, M.; Seidler, S.; Besse, V.; Mathey, M.P.; Nef, J.; Hurni, Y. Stapled diaphragm resection: A new approach to diaphragmatic cytoreductive surgery for advanced-stage ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 279, 88–93. [Google Scholar] [CrossRef]
- Carboni, F.; Federici, O.; Zazza, S.; Sperduti, I.; Valle, M. Feasibility of diaphragmatic interventions in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: A 20-year experience. Eur. J. Surg. Oncol. 2021, 47, 143–148. [Google Scholar] [CrossRef]
- Zapardiel, I.; Peiretti, M.; Zanagnolo, V.; Biffi, R.; Bocciolone, L.; Landoni, F.; Aletti, G.; Colombo, N.; Maggioni, A. Diaphragmatic surgery during primary cytoreduction for advanced ovarian cancer: Peritoneal stripping versus diaphragmatic resection. Int. J. Gynecol. Cancer. 2011, 21, 1698–1703. [Google Scholar] [CrossRef]
- Fanfani, F.; Fagotti, A.; Gallotta, V.; Ercoli, A.; Pacelli, F.; Costantini, B.; Vizzielli, G.; Margariti, P.A.; Garganese, G.; Scambia, G. Upper abdominal surgery in advanced and recurrent ovarian cancer: Role of diaphragmatic surgery. Gynecol. Oncol. 2010, 116, 497–501. [Google Scholar] [CrossRef]
- Passot, G.; Kim, B.J.; Vaudoyer, D.; Kepenekian, V.; Bonnefoy, I.; Bakrin, N.; Cotte, E.; Glehen, O. Digital glissonectomy: A safe perihepatic peritonectomy. Ann. Surg. Oncol. 2016, 23, 3978–3985. [Google Scholar] [CrossRef]
- Han, S.S.; Sugarbaker, P.H. Kocher maneuver to facilitate cytoreduction within the foramen of Winslow. J. Surg. Oncol. 2017, 115, 788–790. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Cytoreductive Surgery and Perioperative Chemotherapy: Textbook and Video Atlas; Cine-Med Publishing: Woodbury, CT, USA, 2013. [Google Scholar]
- Yan, T.D.; Bijelic, L.; Sugarbaker, P.H. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann. Surg. Oncol. 2007, 14, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Pont hepatique (hepatic bridge), an important anatomic structure in cytoreductive surgery. J. Surg. Oncol. 2010, 101, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Ferrandina, G.; Vizzielli, G.; Conte, C.; Lucidi, A.; Costantini, B.; De Rose, A.M.; Di Giorgio, A.; Zannoni, G.F.; Fagotti, A.; et al. Hepatoceliac Lymph Node Involvement in Advanced Ovarian Cancer Patients: Prognostic Role and Clinical Considerations. Ann. Surg. Oncol. 2017, 24, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, C.; Angeles, M.A.; Leray, H.; Tanguy Le Gac, Y.; Ferron, G.; Martinez, A. Transdiaphragmatic and transxiphoid cardiophrenic lymph node resection step-by-step in advanced ovarian cancer. Int. J. Gynecol. Cancer. 2020, 30, 1646–1647. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.A.; Tseng, J.; Murthy, V.; Srivastava, R.; Long Roche, K.C.; Zivanovic, O.; Gardner, G.J.; Chi, D.S.; Park, B.J.; Sonoda, Y. Feasibility, safety and clinical outcomes of cardiophrenic lymph node resection in advanced ovarian cancer. Gynecol. Oncol. 2017, 147, 262–266. [Google Scholar] [CrossRef]
- Lim, M.C.; Lee, H.S.; Jung, D.C.; Choi, J.Y.; Seo, S.S.; Park, S.Y. Pathological diagnosis and cytoreduction of cardiophrenic lymph node and pleural metastasis in ovarian cancer patients using video-assisted thoracic surgery. Ann. Surg. Oncol. 2009, 16, 1990–1996. [Google Scholar] [CrossRef]
- Emilia, M.; Luca, S.; Francesca, B.; Luca, B.; Paolo, S.; Giuseppe, F.; Gianbattista, B.; Carmela, M.; Luigi, M.; Mauro, L. Topical hemostatic agents in surgical practice. Transfus. Apher. Sci. 2011, 45, 305–311. [Google Scholar] [CrossRef]
- Watrowski, R.; Jäger, C.; Forster, J. Improvement of Perioperative Outcomes in Major Gynecological and Gynecologic-Oncological Surgery with Hemostatic Gelatin-Thrombin Matrix. In Vivo 2017, 31, 251–258. [Google Scholar] [CrossRef][Green Version]
- Sargant, N.; Roy, A.; Simpson, S.; Chandrakumaran, K.; Alves, S.; Coakes, J.; Bell, J.; Knight, J.; Wilson, P.; Mohamed, F.; et al. A protocol for management of blood loss in surgical treatment of peritoneal malignancy by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Transfus. Med. 2016, 26, 118–122. [Google Scholar] [CrossRef]
- Hübner, M.; Kusamura, S.; Villeneuve, L.; Al-Niaimi, A.; Alyami, M.; Balonov, K.; Bell, J.; Bristow, R.; Guiral, D.C.; Fagotti, A.; et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced recovery after surgery (ERAS®) Society Recommendations—Part I: Preoperative and intraoperative management. Eur. J. Surg. Oncol. 2020, 46, 2292–2310. [Google Scholar] [CrossRef]
- Pereira, A.; Magrina, J.F.; Magtibay, P.M.; Neto, J.S.; Siufi, D.F.S.; Chang, Y.H.; Perez-Medina, T. Does MIS Play a Role in the Treatment of Advanced Ovarian Cancer? Cancers 2022, 14, 3579. [Google Scholar] [CrossRef] [PubMed]
- Nitecki, R.; Rauh-Hain, J.A.; Melamed, A.; Scambia, G.; Pareja, R.; Coleman, R.L.; Ramirez, P.T.; Fagotti, A. Laparoscopic cytoreduction After Neoadjuvant ChEmotherapy (LANCE). Int. J. Gynecol. Cancer 2020, 30, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Gueli Alletti, S.; Corrado, G.; Cola, E.; Vizza, E.; Vieira, M.; Andrade, C.E.; Tsunoda, A.; Favero, G.; Zapardiel, I.; et al. The International Mission study: Minimally invasive surgery in ovarian neoplasms after neoadjuvant chemotherapy. Int. J. Gynecol. Cancer 2019, 29, 5–9. [Google Scholar] [CrossRef]
- Arjona-Sanchez, A.; Esquivel, J.; Glehen, O.; Passot, G.; Turaga, K.K.; Labow, D.; Rufian-Peña, S.; Morales, R.; van der Speeten, K. A minimally invasive approach for peritonectomy procedures and hyperthermic intraperitoneal chemotherapy (HIPEC) in limited peritoneal carcinomatosis: The American Society of Peritoneal Surface Malignancies (ASPSM) multi-institution analysis. Surg. Endosc. 2019, 33, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Magrina, J.F.; Guardiola, T.C.; Magtibay PM 3rd Kosiorek, H.E.; Magtibay, P.M. Minimally Invasive Surgery for Resection of Diaphragm Metastases in Ovarian Cancer. J. Minim Invasive Gynecol. 2019, 26, 1268–1272. [Google Scholar] [CrossRef]
- Magrina, J.F.; Magtibay, P.M. Robotic Resection of Diaphragm Metastases in Ovarian Cancer: Technical Aspects. J. Minim Invasive Gynecol. 2020, 27, 1417–1422. [Google Scholar] [CrossRef]
- Uccella, S.; Franchi, M.P.; Cianci, S.; Zorzato, P.C.; Bertoli, F.; Alletti, S.G.; Ghezzi, F.; Scambia, G. Laparotomy vs. minimally invasive surgery for ovarian cancer recurrence: A systematic review. Gland Surg. 2020, 9, 1130–1139. [Google Scholar] [CrossRef]
- Gueli Alletti, S.; Capozzi, V.A.; Rosati, A.; De Blasis, I.; Cianci, S.; Vizzielli, G.; Uccella, S.; Gallotta, V.; Fanfani, F.; Fagotti, A.; et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: A systematic review of the literature. Minerva Med. 2019, 110, 341–357. [Google Scholar] [CrossRef]
- Loverro, M.; Ergasti, R.; Conte, C.; Gallitelli, V.; Nachira, D.; Scaglione, G.; Fagotti, A.; Scambia, G.; Gallotta, V. Minimally Invasive Secondary Cytoreductive Surgery for Superficial Celiac and Cardio-Phrenic Isolated Nodal Recurrence of Ovarian Cancer. Ann. Surg. Oncol. 2022, 29, 2603–2604. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, W.; Gao, C.; Zhou, Y.; Xie, Y. Postoperative pulmonary complications and outcomes in cytoreductive surgery for ovarian cancer: A propensity-matched analysis. BMC Anesthesiol. 2022, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.C.; Loewen, R.T.; Aletti, G.; Feitoza, S.S.; Cliby, W. Assessment of outcomes and morbidity following diaphragmatic peritonectomy for women with ovarian carcinoma. Gynecol. Oncol. 2008, 109, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Ditto, A.; Martinelli, F.; Lorusso, D.; Chiappa, V.; Donfrancesco, C.; Di Donato, V.; Indini, A.; Aletti, G.; Raspagliesi, F. Surgical Techniques for Diaphragmatic Resection During Cytoreduction in Advanced or Recurrent Ovarian Carcinoma: A Systematic Review and Meta-analysis. Int. J. Gynecol. Cancer. 2016, 26, 371–380. [Google Scholar] [CrossRef]
- Sinukumar, S.; Naik, S.; Khurjekar, D.; Munde, Y.; Bhosale, S. Pancreaticopleural Fistula After Cytoreductive Surgery and HIPEC for Pseudomyxoma Peritonei-a Rare Presentation and Rare Complication. Indian J. Surg. Oncol. 2020, 11, 174–177. [Google Scholar] [CrossRef]
- Bhatt, A.; Mehta, A.M. Management of Complications of CRS and HIPEC. In Management of Peritoneal Metastases-Cytoreductive Surgery, HIPEC and Beyond; Bhatt, A., Ed.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Cortés-Guiral, D.; Mohamed, F.; Glehen, O.; Passot, G. Prehabilitation of patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal malignancy. Eur. J. Surg. Oncol. 2021, 47, 60–64. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Planchamp, F.; Aytulu, T.; Chiva, L.; Cina, A.; Ergönül, Ö.; Fagotti, A.; Suria, S.; Knapp, P.; Morice, P.; et al. European Society of Gynaecological Oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery. Int. J. Gynecol. Cancer. 2021, 31, 1199–1206. [Google Scholar] [CrossRef]
- Hubner, M.; Kusamura, S.; Villeneuve, L.; Al-Niaimi, A.; Alyami, M.; Balonov, K.; Bell, J.; Altman, A.; Fagotti, A.; Mack, L.; et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced Recovery After Surgery (ERAS) Society Recommendations—Part II: Postoperative management and special considerations. Eur. J. Surg. Oncol. 2020, 46, 2311–2323. [Google Scholar] [CrossRef]
- Dhanis, J.; Keidan, N.; Blake, D.; Rundle, S.; Strijker, D.; van Ham, M.; Pijnenborg, J.M.A.; Smits, A. Prehabilitation to Improve Outcomes of Patients with Gynaecological Cancer: A New Window of Opportunity? Cancers 2022, 14, 3448. [Google Scholar] [CrossRef]
- Diaz-Feijoo, B.; Agusti-Garcia, N.; Sebio, R.; López-Hernández, A.; Sisó, M.; Glickman, A.; Carreras-Dieguez, N.; Fuste, P.; Marina, T.; Martínez-Egea, J.; et al. Feasibility of a Multimodal Prehabilitation Programme in Patients Undergoing Cytoreductive Surgery for Advanced Ovarian Cancer: A Pilot Study. Cancers 2022, 14, 1635. [Google Scholar] [CrossRef]
- Mehta, S.S.; Bhatt, A.; Glehen, O. Cytoreductive Surgery and Peritonectomy Procedures. Indian J. Surg. Oncol. 2016, 7, 139–151. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Taskiran, C. Principles of safe and efficacious upper abdominal surgery. Gynecol. Pelvic Med. 2021, 4, 35. [Google Scholar] [CrossRef]
- Bhatt, A.; Kammar, P.; Sinukumar, S.; Goswami, G.; Mishra, B.; Bhavsar, M.; Shaikh, S.; Bhosale, S.; Aggarwal, D.; Bhorkar, N.; et al. Perioperative outcomes in patients treated with total parietal peritonectomy and multi-visceral resections with or without HIPEC at different time points in the history of advanced ovarian cancer. Eur. J. Gynaecol. Oncol. 2021, 42, 711–720. [Google Scholar]
- Llueca, J.; Herraiz, J.; Catala, C.; Serra, A.; Rivadulla, I.; Escrig, J.; MUAPOS Working Group. Effectiveness and Safety of Cytoreduction Surgery in Advanced Ovarian Cancer: Initial Experience at a University General Hospital. J. Clin. Gynecol. Obstet. 2015, 4, 251–257. Available online: https://www.jcgo.org/index.php/jcgo/article/view/345/191 (accessed on 24 December 2021). [CrossRef]



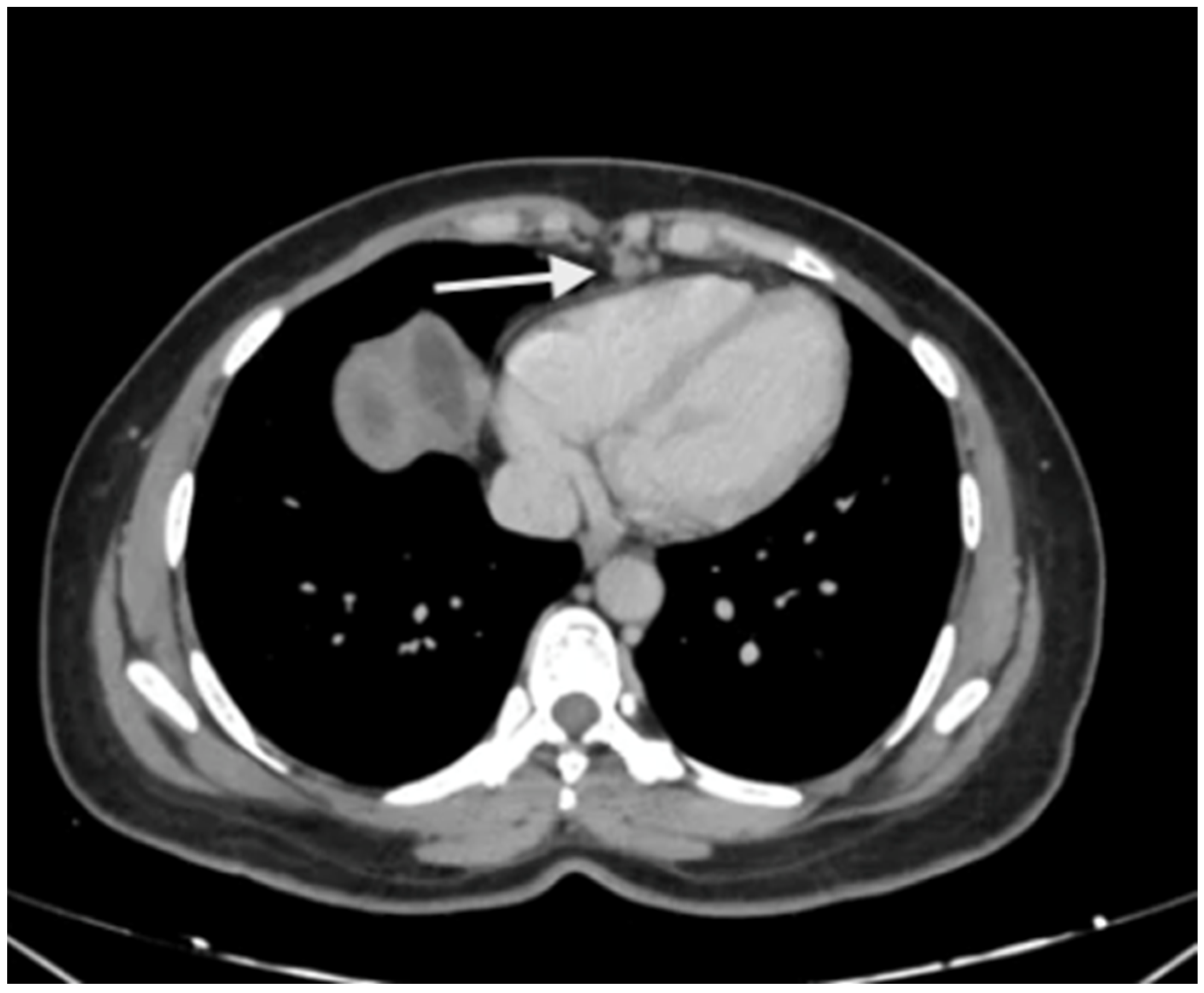

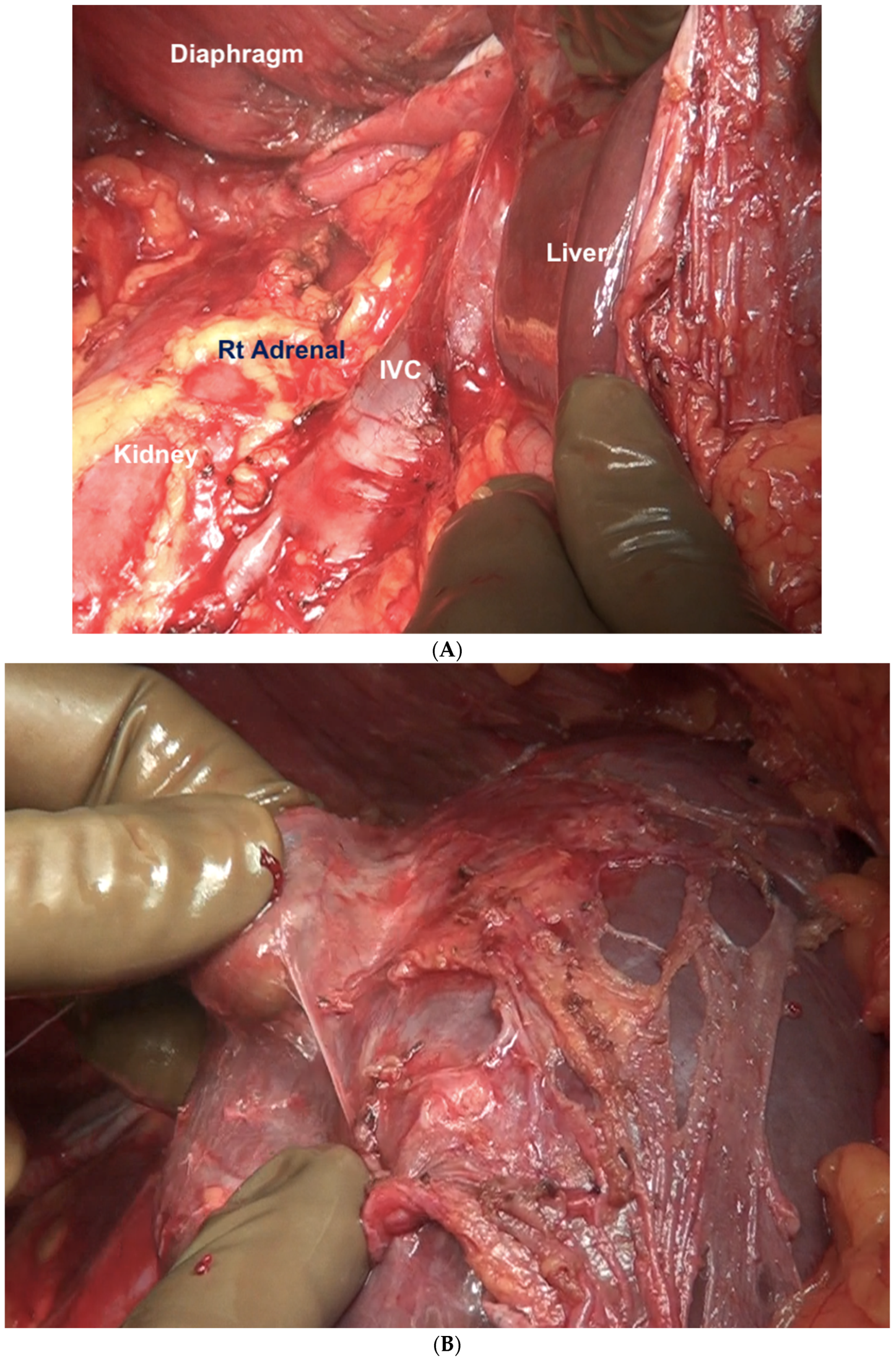
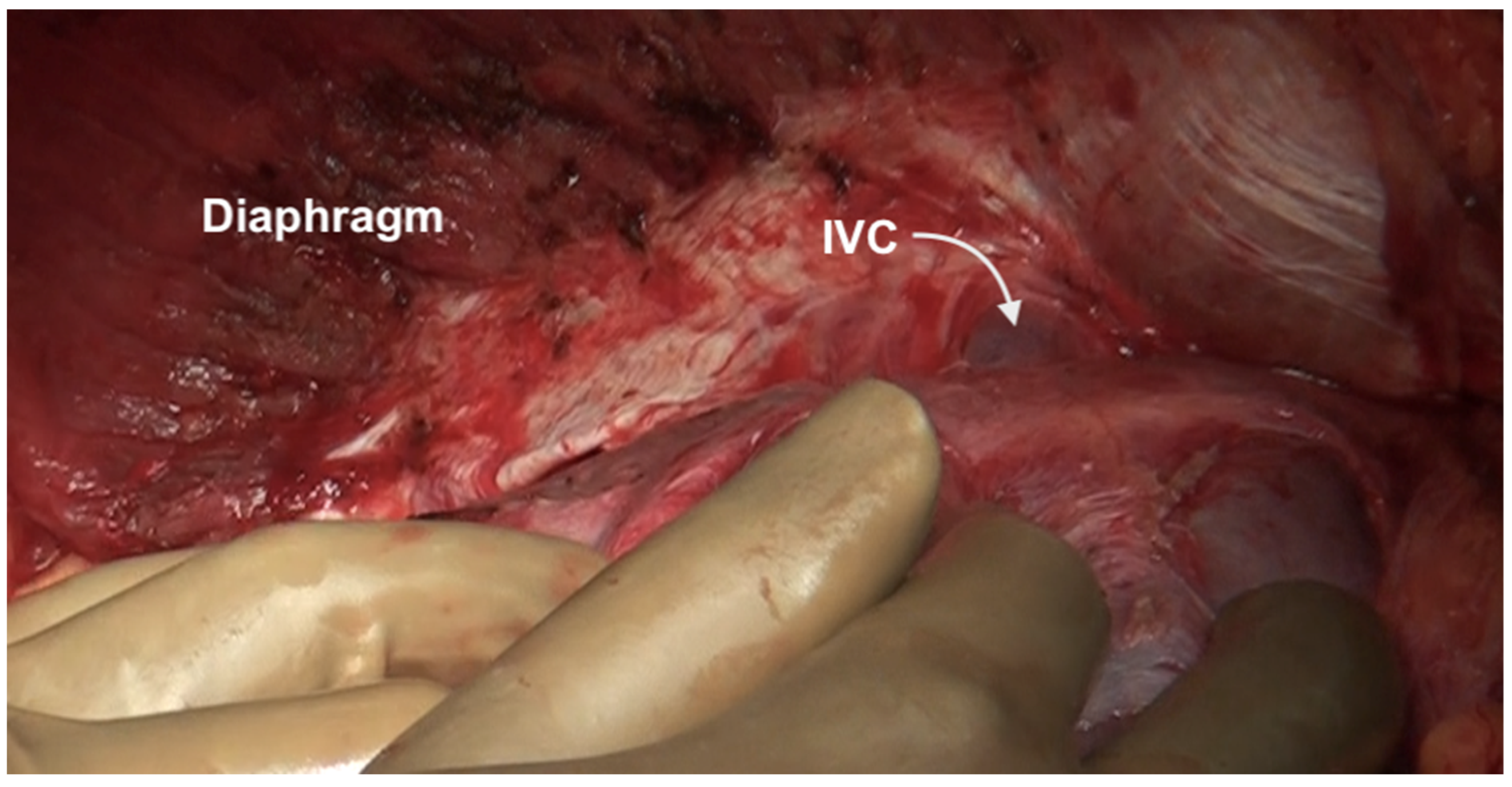



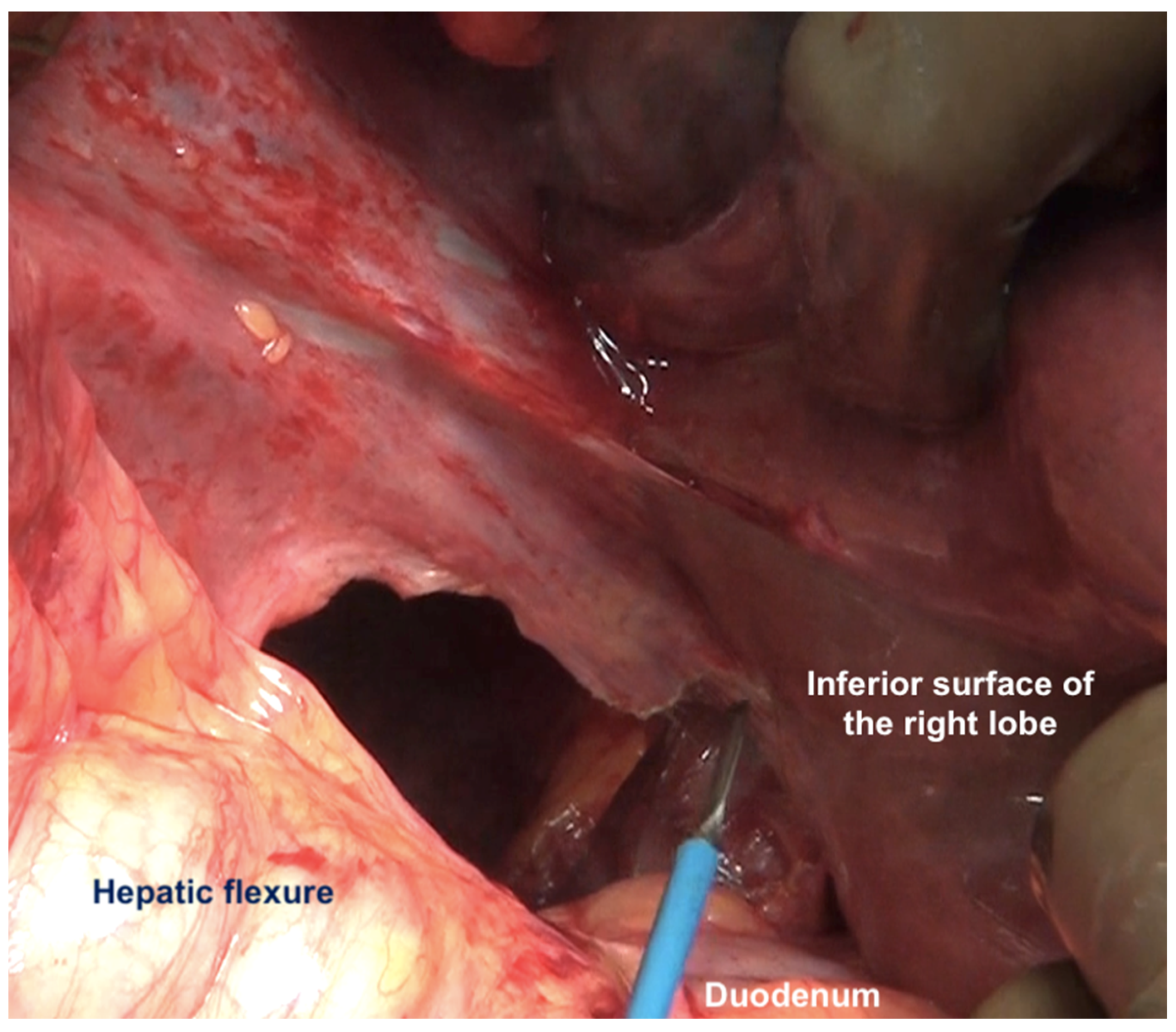






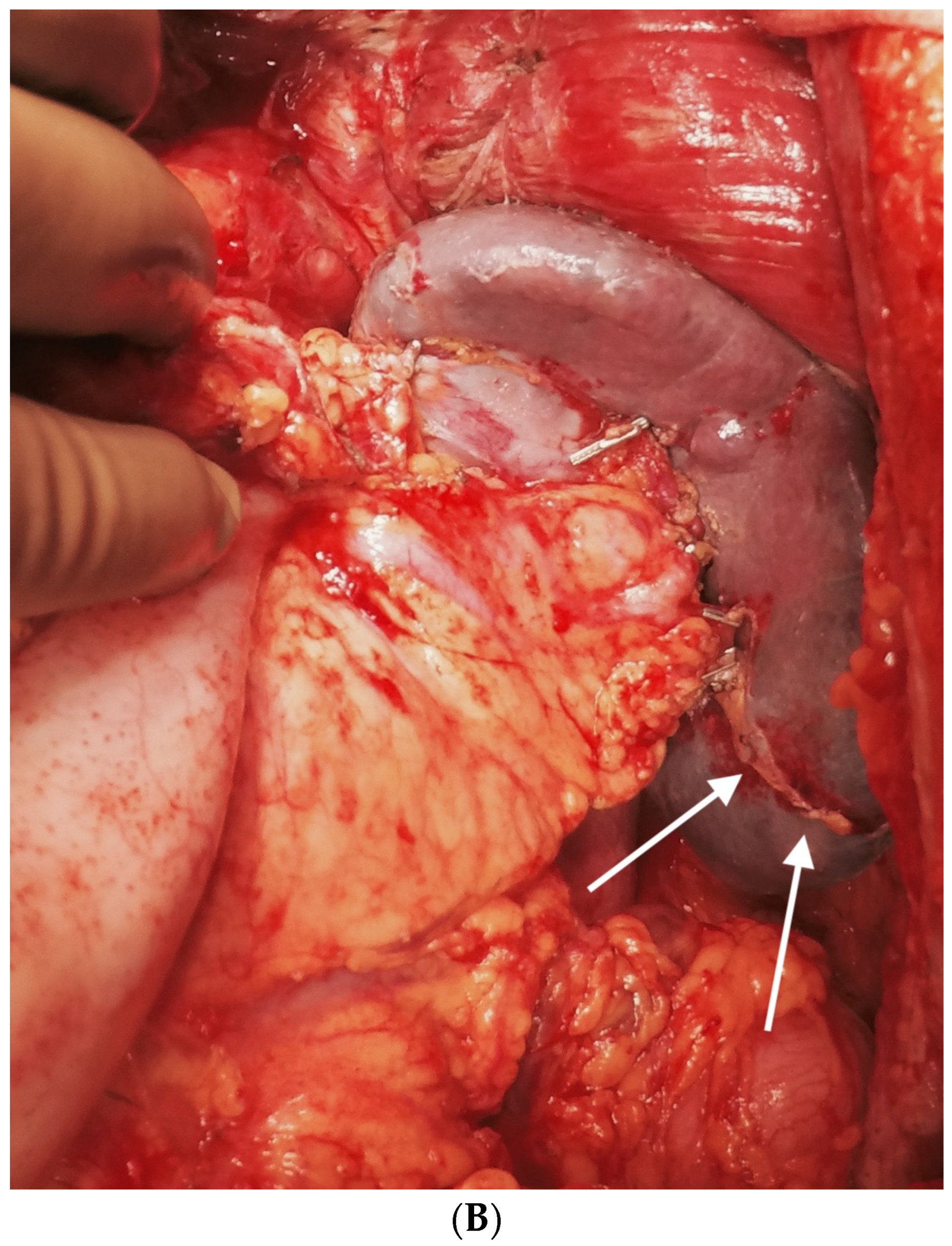
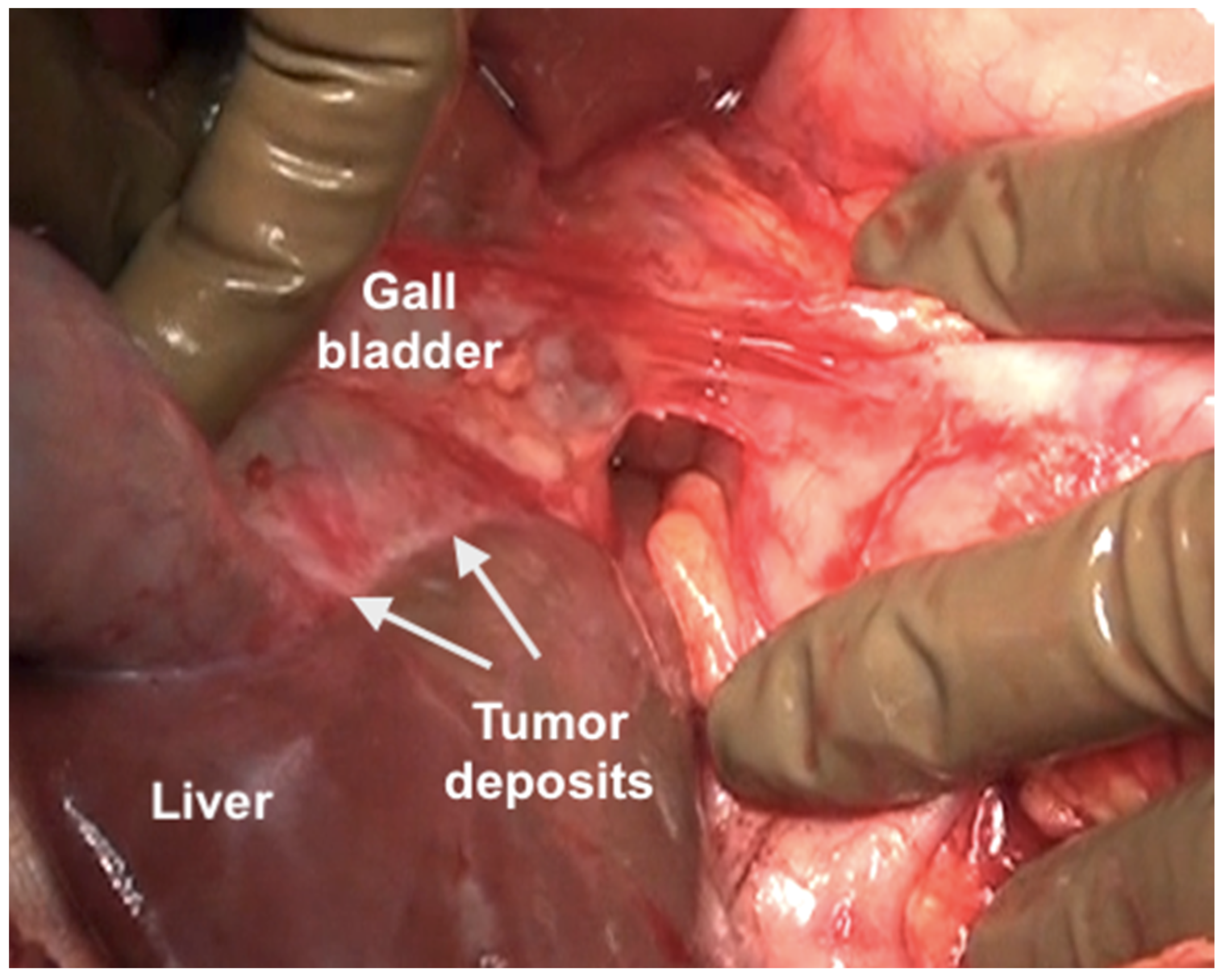


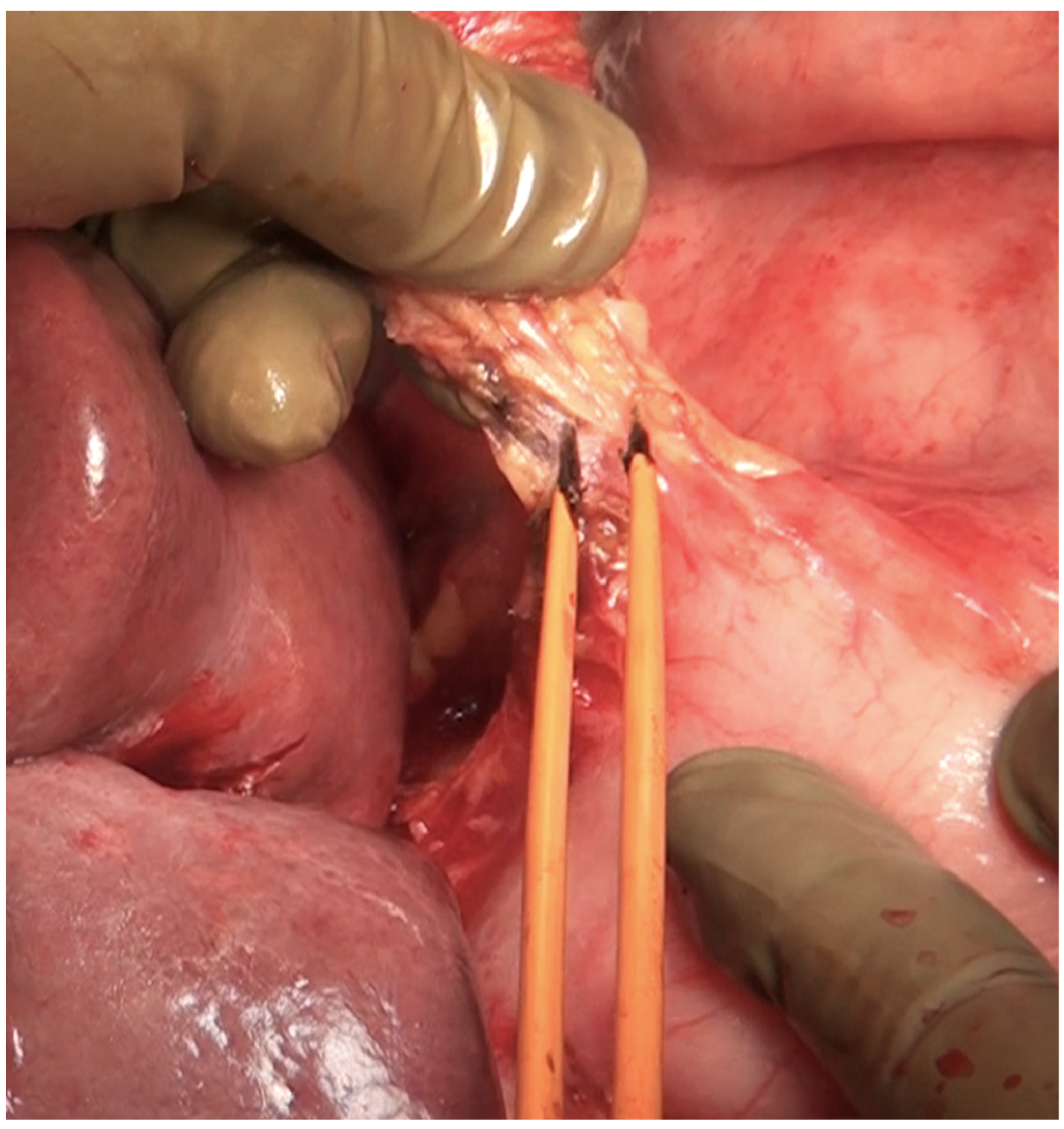
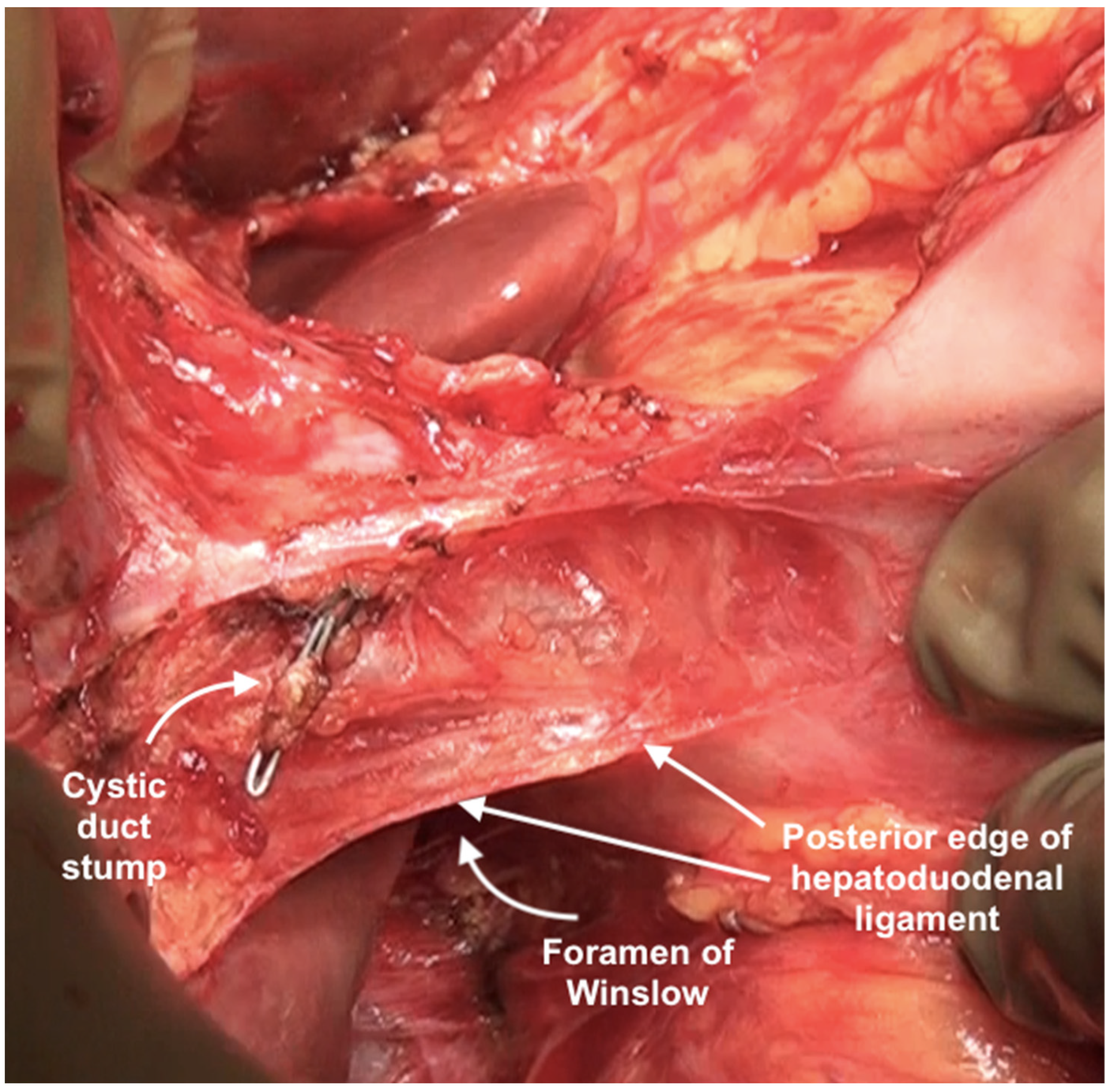


| Region | Peritoneal Region | Boundaries |
|---|---|---|
| 1 | Right subphrenic peritoneum | From the right of the falciform ligament medially, it includes all the peritoneum the under the surface of the right dome, extending inferiorly to the lower pole of the right kidney [43]. Postero-superiorly, it merges with Glisson’s capsule at the superior boundary of the right lobe. The coronary or right triangular ligament has to be divided completely to excise this part of the peritoneum. Postero-medially it is attached to the posteromedial edge of segments 6 and 7. Division of this attachment exposes the retrohepatic inferior vena cava (IVC) [44]. Medially, it follows the inferior edge of segments 5 and 6 to the lateral edge of the porta hepatis and includes the peritoneum on the second and third parts of the duodenum. The sub-hepatic region is Morrison’s pouch, as described below. |
| Morrisons’ pouch | This is the right sub-hepatic space that extends laterally from the medial boundary of the right kidney [43]. It is bounded superiorly by the inferior surface of segments 5 and 6, medially by the lateral edge of the hepatoduodenal ligament, inferiorly, the superior and lateral edge of the duodenum, and inferolaterally, the hepatic flexure. It merges laterally with the right subphrenic peritoneum [44]. | |
| Right Glisson’s capsule | Capsule of the right lobe of the liver (superior and lateral surfaces). Extends from the right side of the falciform’s attachment onto the right lobe’s superior surface and the lateral surface, merging with the attachment of the right subphrenic peritoneum to the liver in that region. | |
| Right inferior Glisson’s capsule | The part of the capsule on the inferior surface of the right lobe and the right caudate lobe. | |
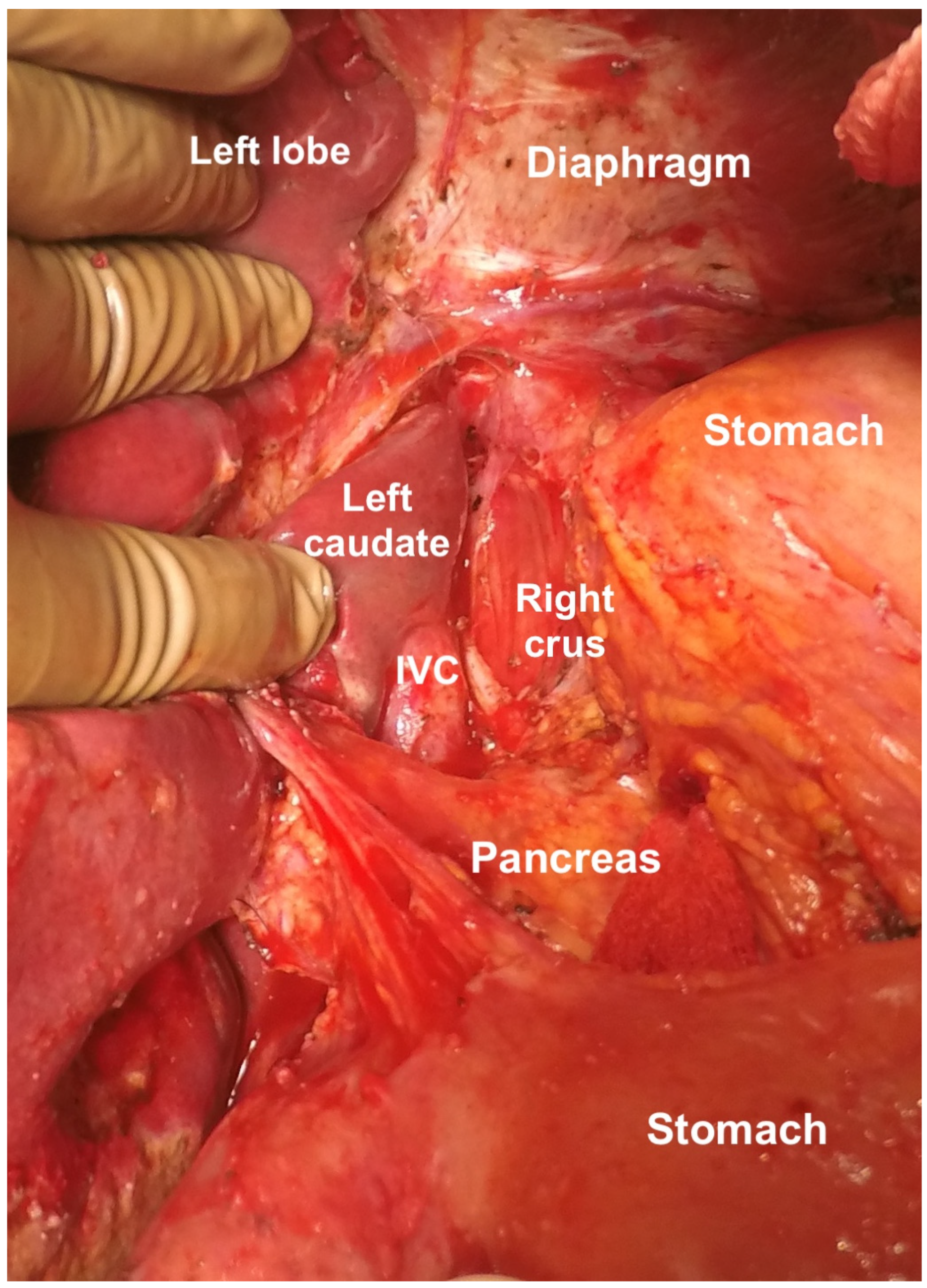
| Foramen of Winslow | The foramen of Winslow, also known as the omental foramen, epiploic foramen and foramen epiploicum (Latin), is a foramen connecting the greater sac or the general cavity (of the abdomen), and the lesser sac, the omental bursa. It is bounded by the free edge of the lesser omentum anteriorly that contains the common bile duct, the hepatic artery and the portal vein; the peritoneum covering the anterior surface of the inferior vena cava posteriorly; the peritoneum covering the caudate lobe superiorly; and the peritoneum covering the first part of the duodenum inferiorly. | |
| 2 | Falciform ligament | It is a fold of fibrofatty tissue extending from the umbilicus along the inferior surface of the anterior abdominal wall to the diaphragm. Inferiorly, it forms the umbilical round ligament in the umbilical fissure containing the obliterated umbilical vein. |
| Tissue in the umbilical fissure | This is a band of tissue containing the obliterated umbilical vein that merges superiorly with the falciform ligament and inferiorly with the hepatoduodenal ligament. | |
| Left central diaphragmatic peritoneum- | From the left edge of the falciform ligament till the left lateral boundary of the esophageal hiatus and inferiorly, it merges with the peritoneal reflection on the superior edge of the left lobe of the liver. | |
| Left Glisson’s capsule- | Capsule on the superior and inferolateral surface of the left lobe that lies to the left of the falciform ligament | |
| Hepatoduodenal ligament | This is the peritoneum overlying the porta hepatis extending from the lateral border of the common bile duct to the medial border of the portal vein and the lower end of the umbilical ligament to the superior border of the first part of the duodenum. The common bile duct, the hepatic artery, and the portal vein are encased in this peritoneal fold. | |
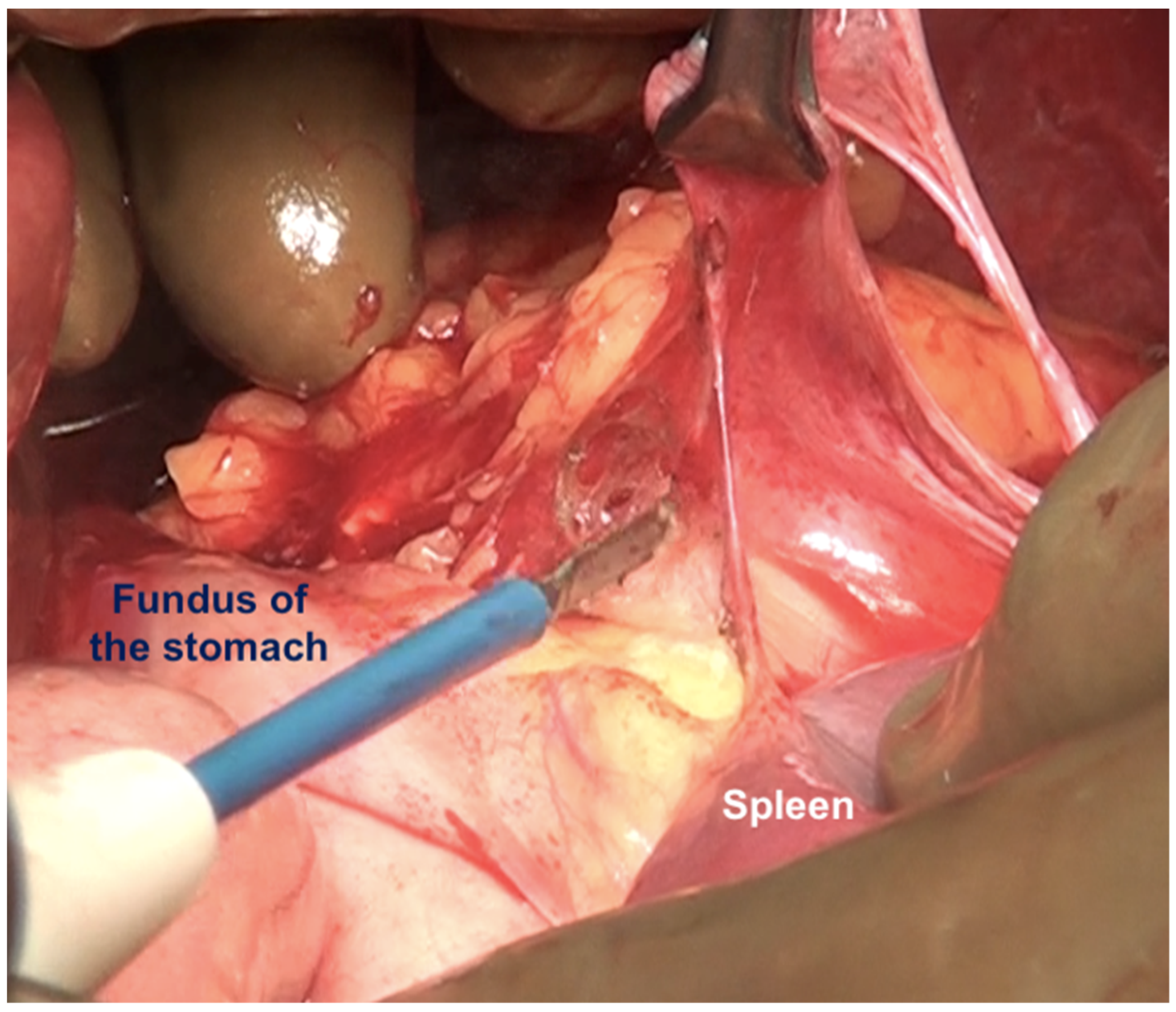
| Lesser omentum | The lesser omentum extends from the medial boundary of the hepatoduodenal ligament inferiorly to the left coronary ligament superiorly. It is attached inferomedially to the lesser curve and superiorly to the inferior surface of the left lobe overhanging the caudate. The left coronary ligament must be divided completely to excise the lesser omentum. The gastric arcade is preserved without gross tumor deposits in this region. | |
| Lesser sac superior recess | This is part of the lesser sac lying superior to the left coronary ligament. The caudate lobe is reflected laterally to expose this region. This region is bounded by the IVC laterally, and the lesser curve medially, and its floor is formed by the IVC and the right crus of the diaphragm. Superiorly it merges with the central diaphragmatic peritoneum. | |
| Lesser sac inferior recess | This includes the anterior leaf of the transverse mesocolon, the pancreatic capsule, and the gastro-pancreatic fold of the peritoneum. The capsule is removed over the entire pancreas, head, body, and tail. | |
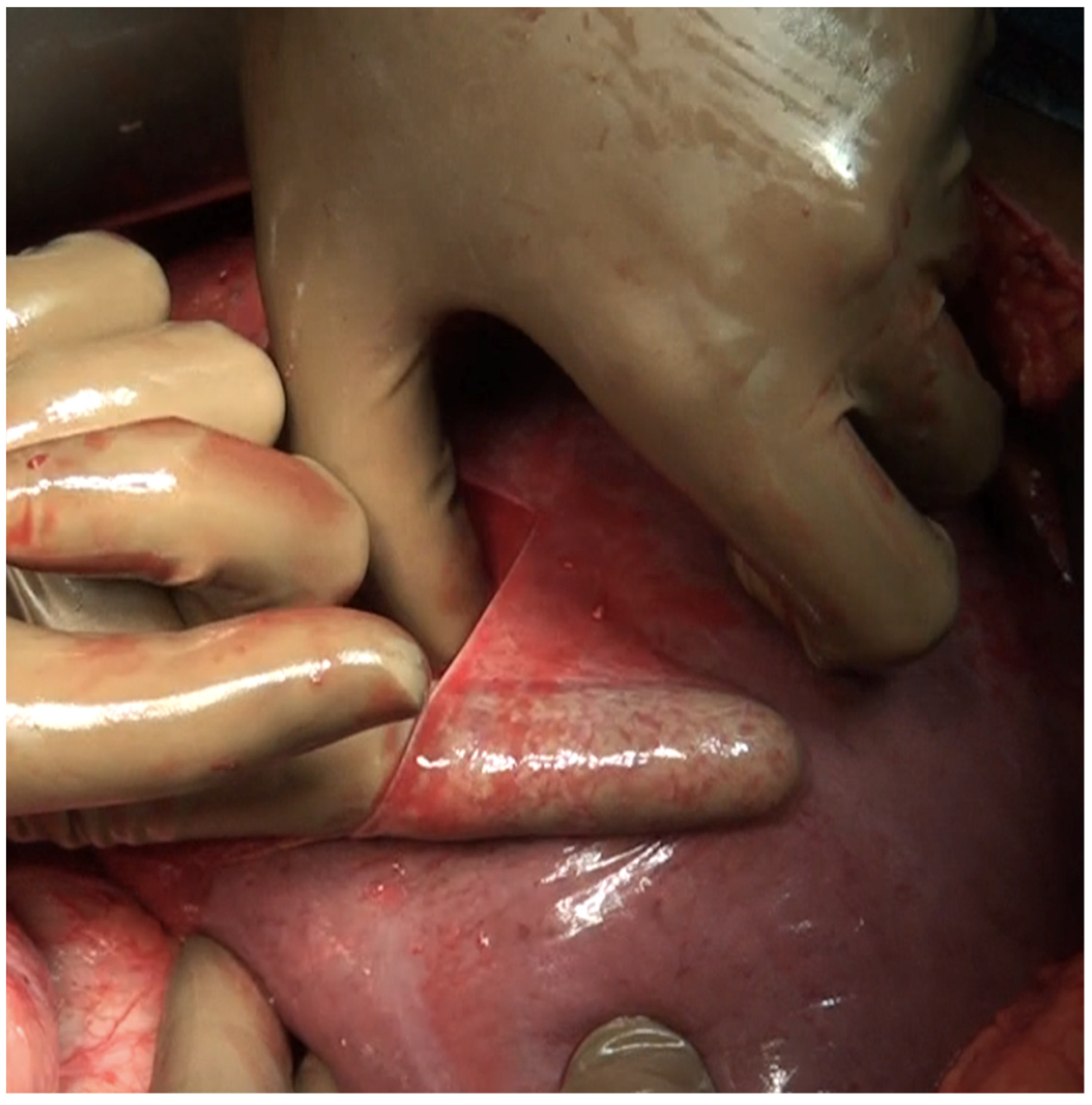
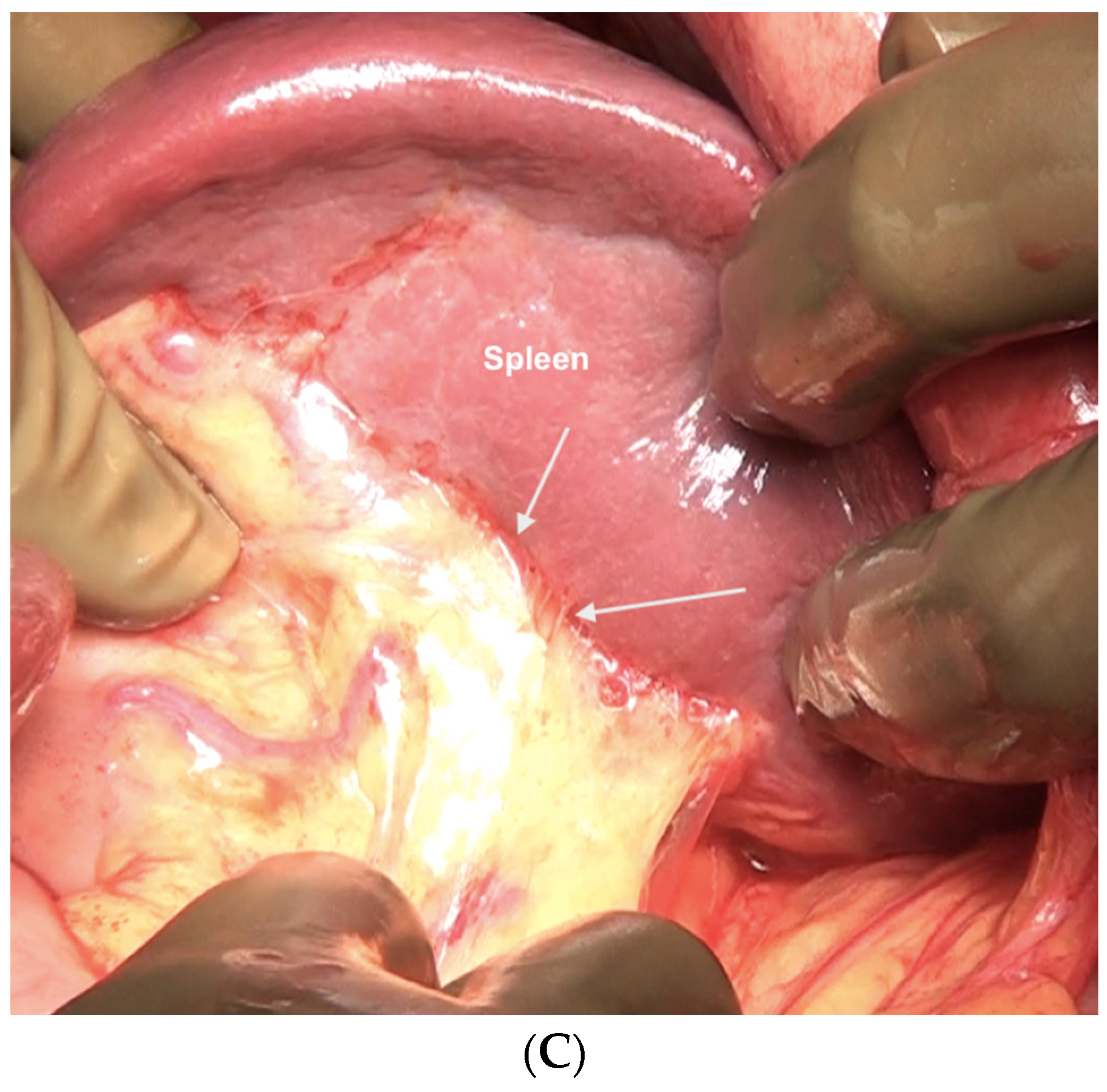
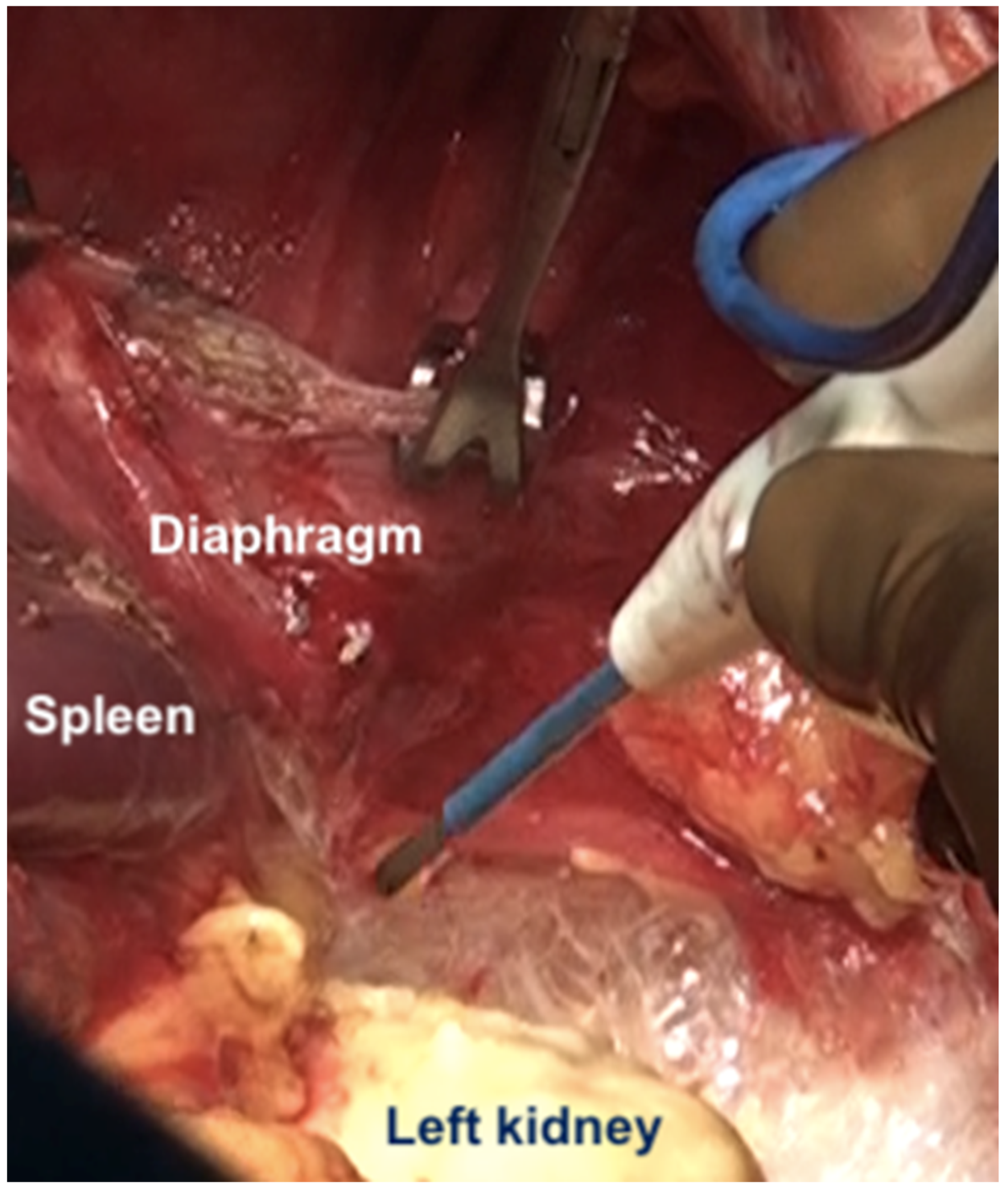
| 3 | Left subphrenic peritoneum | Extends from the midline anteriorly to the left lateral side to include all the peritoneum on the undersurface of the left dome of the diaphragm, merging postero-inferiorly with the upper end of the left antero-parietal peritoneum, superomedially the lateral edge of the abdominal esophagus and fundus, and along the lateral edge of the spleen inferomedially. Removal of the peritoneum from the spleen should extend right up to the hilum posteriorly and expose the splenic hilar vessels. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandoria, G.; Bhatt, A.; Mehta, S.; Glehen, O. Upper-Abdominal Cytoreduction for Advanced Ovarian Cancer—Therapeutic Rationale, Surgical Anatomy and Techniques of Cytoreduction. Surg. Tech. Dev. 2023, 12, 1-33. https://doi.org/10.3390/std12010001
Bhandoria G, Bhatt A, Mehta S, Glehen O. Upper-Abdominal Cytoreduction for Advanced Ovarian Cancer—Therapeutic Rationale, Surgical Anatomy and Techniques of Cytoreduction. Surgical Techniques Development. 2023; 12(1):1-33. https://doi.org/10.3390/std12010001
Chicago/Turabian StyleBhandoria, Geetu, Aditi Bhatt, Sanket Mehta, and Olivier Glehen. 2023. "Upper-Abdominal Cytoreduction for Advanced Ovarian Cancer—Therapeutic Rationale, Surgical Anatomy and Techniques of Cytoreduction" Surgical Techniques Development 12, no. 1: 1-33. https://doi.org/10.3390/std12010001
APA StyleBhandoria, G., Bhatt, A., Mehta, S., & Glehen, O. (2023). Upper-Abdominal Cytoreduction for Advanced Ovarian Cancer—Therapeutic Rationale, Surgical Anatomy and Techniques of Cytoreduction. Surgical Techniques Development, 12(1), 1-33. https://doi.org/10.3390/std12010001






