Acute Promyelocytic Leukemia: Pathophysiology, Diagnosis and Clinical Management
Abstract
1. Introduction
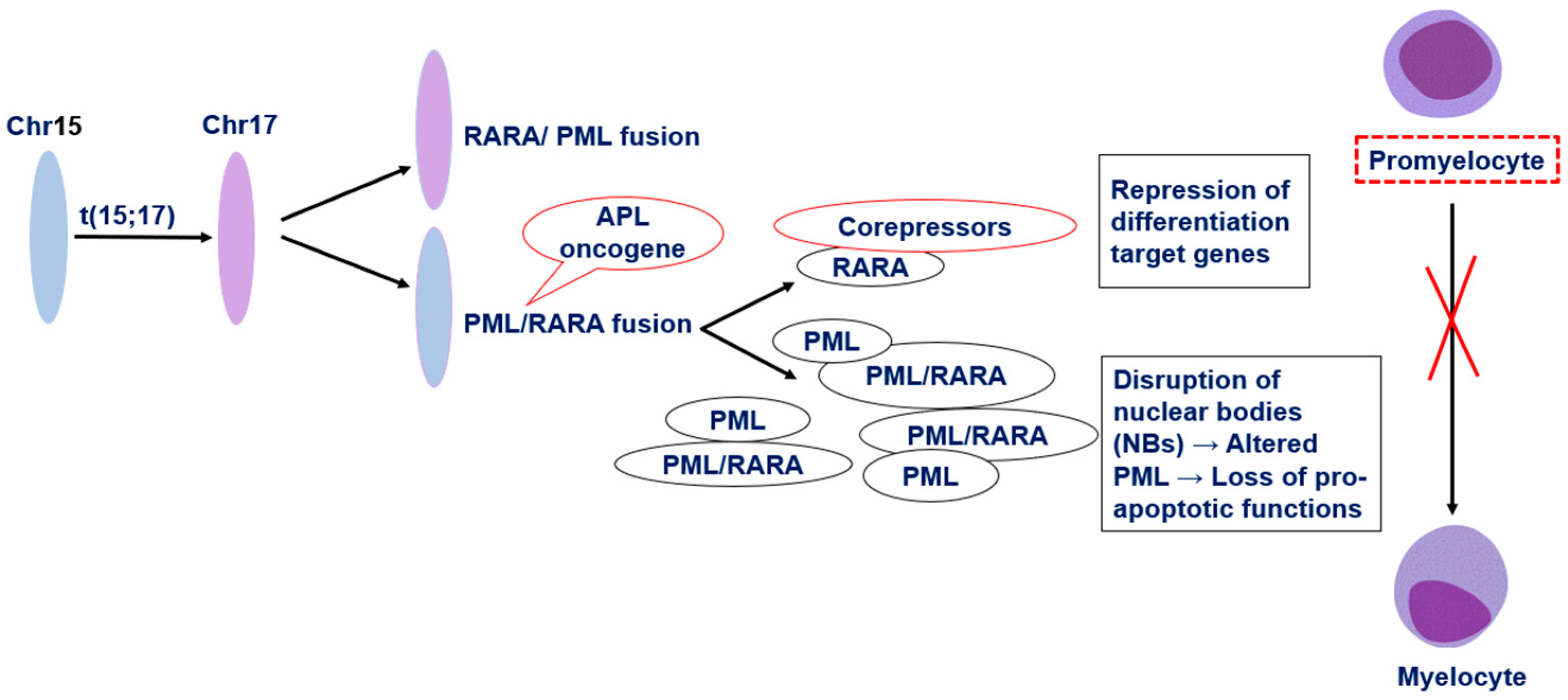
2. Pathophysiology of Acute Promyelocytic Leukemia
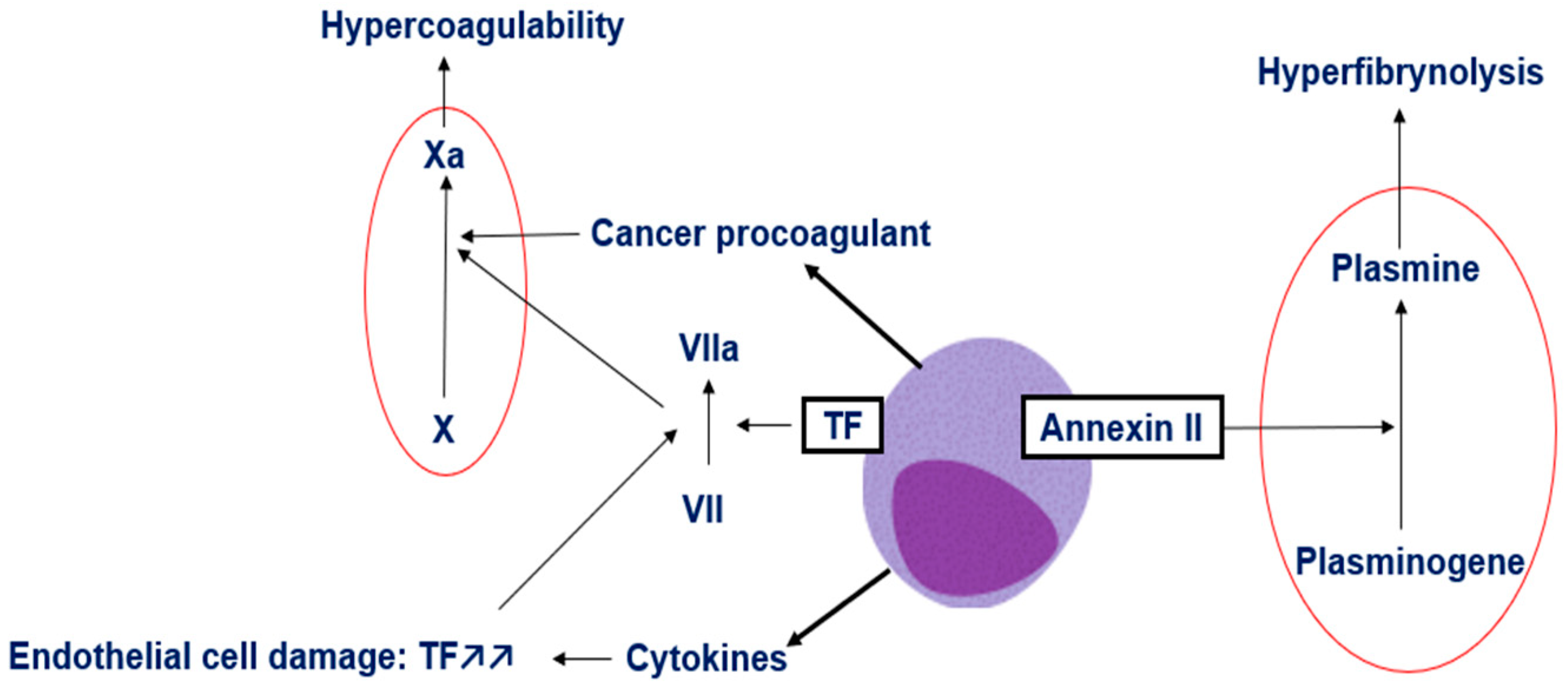
3. Mechanisms of Coagulopathy in APL
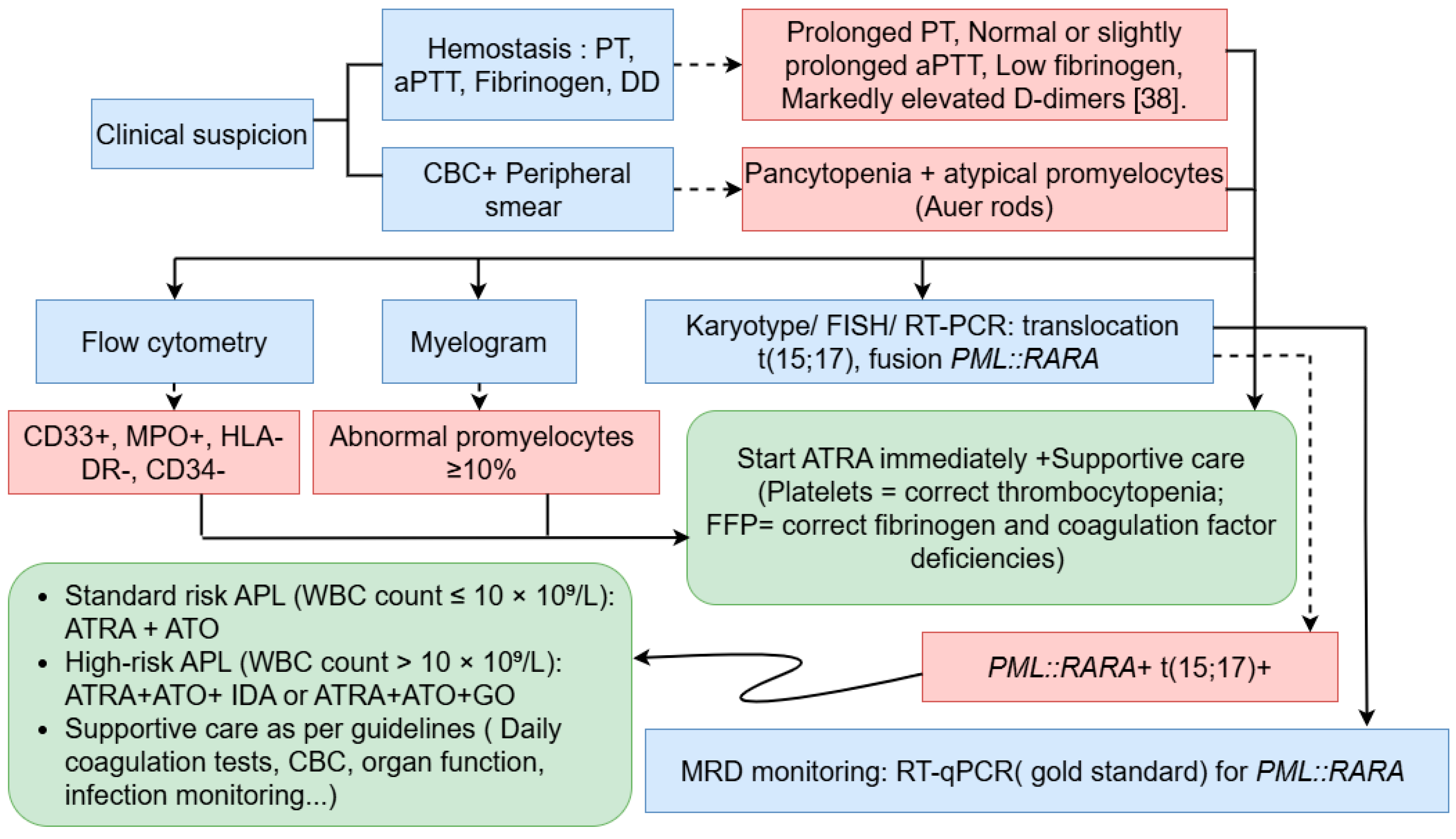
4. Acute Promyelocytic Leukemia Diagnosis
4.1. Clinical and Morphological Assessment
4.2. Immunophenotyping by Flow Cytometry
4.3. Coagulation Studies
4.4. Molecular and Cytogenetic Analyses
5. Clinical Management
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bidikian, A.; Bewersdorf, J.P.; Kewan, T.; Stahl, M.; Zeidan, A.M. Acute Promyelocytic Leukemia in the Real World: Understanding Outcome Differences and How We Can Improve Them. Cancers 2024, 16, 4092. [Google Scholar] [CrossRef]
- Santana-Hernández, J.; Corona-Rivera, A.; Mendoza-Maldonado, L.; Santana-Bejarano, U.F.; Cuero-Quezada, I.; Marquez-Mora, A.; Serafín-Saucedo, G.; Brukman-Jiménez, S.A.; Corona-Rivera, R.; Ortuño-Sahagún, D.; et al. Acute Promyelocytic Leukemia with PML/RARA (Bcr1, Bcr2 and Bcr3) Transcripts in a Pediatric Patient. Oncol. Lett. 2024, 27, 114. [Google Scholar] [CrossRef]
- Eckardt, J.-N.; Schmittmann, T.; Riechert, S.; Kramer, M.; Sulaiman, A.S.; Sockel, K.; Kroschinsky, F.; Schetelig, J.; Wagenführ, L.; Schuler, U.; et al. Deep Learning Identifies Acute Promyelocytic Leukemia in Bone Marrow Smears. BMC Cancer 2022, 22, 201. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Yu, W.; Jin, J. Current Views on the Genetic Landscape and Management of Variant Acute Promyelocytic Leukemia. Biomark. Res. 2021, 9, 33. [Google Scholar] [CrossRef]
- Iyer, S.G.; Elias, L.; Stanchina, M.; Watts, J. The Treatment of Acute Promyelocytic Leukemia in 2023: Paradigm, Advances, and Future Directions. Front. Oncol. 2023, 12, 1062524. [Google Scholar] [CrossRef]
- Xi, X.-D.; Mao, J.-H.; Wang, K.-K.; Caen, J.; Chen, S.-J. Hémorragie dans la leucémie aiguë promyélocytaire et au-delà: Les rôles du facteur tissulaire et des mécanismes de régulation sous-jacents. Bull. Acad. Natl. Méd. 2023, 207, 408–415. [Google Scholar] [CrossRef]
- Hermsen, J.; Hambley, B. The Coagulopathy of Acute Promyelocytic Leukemia: An Updated Review of Pathophysiology, Risk Stratification, and Clinical Management. Cancers 2023, 15, 3477. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, A.; DeLoughery, T.G. Bleeding and Thrombosis in Acute Promyelocytic Leukemia. Am. J. Hematol. 2012, 87, 596–603. [Google Scholar] [CrossRef]
- Sabljic, N.; Thachil, J.; Pantic, N.; Mitrovic, M. Hemorrhage in Acute Promyelocytic Leukemia—Fibrinolysis in Focus. Res. Pract. Thromb. Haemost. 2024, 8, 102499. [Google Scholar] [CrossRef] [PubMed]
- Odetola, O.; Tallman, M.S. How to Avoid Early Mortality in Acute Promyelocytic Leukemia. Hematology 2023, 2023, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Kankhaw, S.; Owattanapanich, W.; Promsuwicha, O.; Thong-ou, T.; Ruchutrakool, T.; Khuhapinant, A.; Paisooksantivatana, K.; Kungwankiattichai, S. Immunophenotypic Profiling of Acute Promyelocytic Leukemia: Insights From a Large Cohort. Cancer Rep. 2025, 8, e70198. [Google Scholar] [CrossRef] [PubMed]
- Klausner, M.; Stinnett, V.; Ghabrial, J.; Morsberger, L.; DeMetrick, N.; Long, P.; Zhu, J.; Smith, K.; James, T.; Adams, E.; et al. Optical Genome Mapping Reveals Complex and Cryptic Rearrangement Involving PML::RARA Fusion in Acute Promyelocytic Leukemia. Genes 2024, 15, 1402. [Google Scholar] [CrossRef] [PubMed]
- Voisset, E.; Moravcsik, E.; Stratford, E.W.; Jaye, A.; Palgrave, C.J.; Hills, R.K.; Salomoni, P.; Kogan, S.C.; Solomon, E.; Grimwade, D. Pml Nuclear Body Disruption Cooperates in APL Pathogenesis and Impairs DNA Damage Repair Pathways in Mice. Blood 2018, 131, 636–648. [Google Scholar] [CrossRef]
- Shima, Y.; Honma, Y.; Kitabayashi, I. PML-RARα and Its Phosphorylation Regulate PML Oligomerization and HIPK2 Stability. Cancer Res. 2013, 73, 4278–4288. [Google Scholar] [CrossRef]
- Tan, Y.; Li, J.; Zhang, S.; Zhang, Y.; Zhuo, Z.; Ma, X.; Yin, Y.; Jiang, Y.; Cong, Y.; Meng, G. Cryo-EM Structure of PML RBCC Dimer Reveals CC-Mediated Octopus-like Nuclear Body Assembly Mechanism. Cell Discov. 2024, 10, 118. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Chale, R.S.; Abad, A.C.; Schally, A.V. Acute Promyelocytic Leukemia (APL): A Review of the Literature. Oncotarget 2020, 11, 992–1003. [Google Scholar] [CrossRef]
- Zhang, A.; Qiu, S. Advances in RARα Fusion Genes in Acute Promyelocytic Leukemia. Exp. Hematol. 2025, 149, 104822. [Google Scholar] [CrossRef]
- Chaudhry, R.; Killeen, R.B.; Babiker, H.M. Physiology, Coagulation Pathways. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Park, S.; Park, J.K. Back to Basics: The Coagulation Pathway. Blood Res. 2024, 59, 35. [Google Scholar] [CrossRef]
- Liquori, A.; Ibañez, M.; Sargas, C.; Sanz, M.Á.; Barragán, E.; Cervera, J. Acute Promyelocytic Leukemia: A Constellation of Molecular Events around a Single PML-RARA Fusion Gene. Cancers 2020, 12, 624. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, J.; Yan, L.; Jin, B.; Hou, W.; Cao, F.; Li, H.; Zhou, J.; Zhang, Y. Tissue Factor–Bearing Microparticles Are a Link between Acute Promyelocytic Leukemia Cells and Coagulation Activation: A Human Subject Study. Ann. Hematol. 2021, 100, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Hambley, B.C.; Tomuleasa, C.; Ghiaur, G. Coagulopathy in Acute Promyelocytic Leukemia: Can We Go Beyond Supportive Care? Front. Med. 2021, 8, 722614. [Google Scholar] [CrossRef]
- Taylor, J.; Nonas, S. A Different Killer: Early Death in Acute Promyelocytic Leukemia Presenting with Septic Shock. CHEST 2020, 158, A903. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating Morphologic, Clinical, and Genomic Data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Salman, H. Comparative Analysis of AML Classification Systems: Evaluating the WHO, ICC, and ELN Frameworks and Their Distinctions. Cancers 2024, 16, 2915. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, T.; Sharma, P. Auer Rods and Faggot Cells: A Review of the History, Significance and Mimics of Two Morphological Curiosities of Enduring Relevance. Eur. J. Haematol. 2023, 110, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ladak, N.; Liu, Y.; Burke, A.; Lin, O.; Chan, A. Acute Promyelocytic Leukemia: A Rare Presentation without Systemic Disease. Hum. Pathol. Rep. 2024, 37, 300753. [Google Scholar] [CrossRef]
- Cheli, E.; Chevalier, S.; Kosmider, O.; Eveillard, M.; Chapuis, N.; Plesa, A.; Heiblig, M.; Andre, L.; Pouget, J.; Mossuz, P.; et al. Diagnosis of Acute Promyelocytic Leukemia Based on Routine Biological Parameters Using Machine Learning. Haematologica 2022, 107, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Lan, H.; Yi, D.; Xian, B.; Zidan, L.; Li, J.; Liao, Z. Flow Cytometry in Acute Myeloid Leukemia and Detection of Minimal Residual Disease. Clin. Chim. Acta 2025, 564, 119945. [Google Scholar] [CrossRef]
- Fang, H.; Wang, S.A.; Hu, S.; Konoplev, S.N.; Mo, H.; Liu, W.; Zuo, Z.; Xu, J.; Jorgensen, J.L.; Yin, C.C.; et al. Acute Promyelocytic Leukemia: Immunophenotype and Differential Diagnosis by Flow Cytometry. Cytom. Part B Clin. Cytom. 2022, 102, 283–291. [Google Scholar] [CrossRef]
- Wen, Z.; Xue, X.; Li, S.; Liu, Y.; Jin, Y.; Jiang, N.; Liao, H. Measurable Residual Disease Analysis by Flow Cytometry and Correlation With Molecular Measurable Residual Disease in Acute Promyelocytic Leukemia: A Real-World Prospective Study. Arch. Pathol. Lab. Med. 2025, 149, 262–270. [Google Scholar] [CrossRef]
- Shahmarvand, N.; Oak, J.S.; Cascio, M.J.; Alcasid, M.; Goodman, E.; Medeiros, B.C.; Arber, D.A.; Zehnder, J.L.; Ohgami, R.S. A Study of Disseminated Intravascular Coagulation in Acute Leukemia Reveals Markedly Elevated D-Dimer Levels Are a Sensitive Indicator of Acute Promyelocytic Leukemia. Int. J. Lab. Hematol. 2017, 39, 375–383. [Google Scholar] [CrossRef]
- De Figueiredo-Pontes, L.L.; Catto, L.F.B.; Chauffaille, M.D.L.L.F.; Pagnano, K.B.B.; Madeira, M.I.A.; Nunes, E.C.; Hamerschlak, N.; De Andrade Silva, M.C.; Carneiro, T.X.; Bortolheiro, T.C.; et al. Diagnosis and Management of Acute Promyelocytic Leukemia: Brazilian Consensus Guidelines 2024 on Behalf of the Brazilian Association of Hematology, Hemotherapy and Cellular Therapy. Hematol. Transfus. Cell Ther. 2024, 46, 553–569. [Google Scholar] [CrossRef]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.-J.; et al. Management of Acute Promyelocytic Leukemia: Updated Recommendations from an Expert Panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DiNardo, C.D.; Kadia, T.M.; Daver, N.G.; Altman, J.K.; Stein, E.M.; Jabbour, E.; Schiffer, C.A.; Lang, A.; Ravandi, F. Acute Myeloid Leukemia Management and Research in 2025. CA A Cancer J. Clin. 2025, 75, 46–67. [Google Scholar] [CrossRef] [PubMed]
- St. Louis, H.; Protopsaltis, N.; Ames, E.; Tanaka, T.; Song, W. Detection of Cytogenetically Cryptic PML-RARA Fusion in Acute Promyelocytic Leukemia by Rapid next Generation Sequencing. NPJ Precis. Oncol. 2025, 9, 279. [Google Scholar] [CrossRef]
- Chandhok, N.S.; Sekeres, M.A. Measurable Residual Disease in Hematologic Malignancies: A Biomarker in Search of a Standard. eClinicalMedicine 2025, 86, 103348. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, U.; Mathews, V. Evolving Chemotherapy Free Regimens for Acute Promyelocytic Leukemia. Front. Oncol. 2021, 11, 621566. [Google Scholar] [CrossRef]
- Woods, A.C.; Norsworthy, K.J. Differentiation Syndrome in Acute Leukemia: APL and Beyond. Cancers 2023, 15, 4767. [Google Scholar] [CrossRef] [PubMed]
- Cicconi, L.; Bisegna, M.; Gurnari, C.; Fanciullo, D.; Piciocchi, A.; Marsili, G.; Minotti, C.; Scalzulli, E.; Mandelli, B.; Guarnera, L.; et al. Leucocytosis during Induction Therapy with All-Trans-Retinoic Acid and Arsenic Trioxide in Acute Promyelocytic Leukaemia Predicts Differentiation Syndrome and Treatment-Related Complications. Br. J. Haematol. 2024, 205, 1727–1733. [Google Scholar] [CrossRef]
- Platzbecker, U.; Adès, L.; Montesinos, P.; Ammatuna, E.; Fenaux, P.; Baldus, C.; Bernardi, M.; Berthon, C.; Bocchia, M.; Bonmati, C.; et al. Arsenic Trioxide and All-Trans Retinoic Acid Combination Therapy for the Treatment of High-Risk Acute Promyelocytic Leukemia: Results From the APOLLO Trial. J. Clin. Oncol. 2025, 43, 3160–3169. [Google Scholar] [CrossRef] [PubMed]
- Voso, M.T.; Guarnera, L.; Lehmann, S.; Döhner, K.; Döhner, H.; Platzbecker, U.; Russell, N.; Dillon, R.; Thomas, I.; Ossenkoppele, G.; et al. Acute Promyelocytic Leukemia: Long-Term Outcomes from the HARMONY Project. Blood 2025, 145, 234–243. [Google Scholar] [CrossRef]
- Chin, K.-K.; Tallman, M.S. Curative Strategies for High-Risk Acute Promyelocytic Leukemia. Curr. Opin. Oncol. 2025, 37, 633–640. [Google Scholar] [CrossRef]
- Yilmaz, M.; Kantarjian, H.; Ravandi, F. Acute Promyelocytic Leukemia Current Treatment Algorithms. Blood Cancer J. 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, H.; Dong, F.; Li, Y.; Liu, Q.; Hu, Y.; Zhang, Y.; Jin, J.; Hu, J.; Liu, T.; et al. Recommendations on Minimal Residual Disease Monitoring in Acute Promyelocytic Leukemia Treated with Combination of All- Trans Retinoic Acid and Arsenic Trioxide. Blood 2023, 142, 2946. [Google Scholar] [CrossRef]
- Sasaki, K.; Ravandi, F.; Kadia, T.; DiNardo, C.D.; Yilmaz, M.; Short, N.; Jabbour, E.; Patel, K.P.; Loghavi, S.; Pierce, S.; et al. Outcome of Patients with Relapsed Acute Promyelocytic Leukemia. Clin. Lymphoma Myeloma Leuk. 2024, 24, 375–381. [Google Scholar] [CrossRef]
- Mohty, R.; Reljic, T.; Yassine, F.; Kettaneh, C.; Al-Husni, D.; Keller, K.; Badar, T.; Murthy, H.; Foran, J.; Kumar, A.; et al. Efficacy of Autologous and Allogeneic Hematopoietic Cell Transplantation in Adults with Acute Promyelocytic Leukemia: Results of a Systematic Review and Meta-Analysis. Transplant. Cell. Ther. 2024, 30, 599.e1–599.e10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abddaoui, M.; Aghlallou, Y.; Tlemçani, I.; Amrani Hassani, M. Acute Promyelocytic Leukemia: Pathophysiology, Diagnosis and Clinical Management. Hematol. Rep. 2025, 17, 66. https://doi.org/10.3390/hematolrep17060066
Abddaoui M, Aghlallou Y, Tlemçani I, Amrani Hassani M. Acute Promyelocytic Leukemia: Pathophysiology, Diagnosis and Clinical Management. Hematology Reports. 2025; 17(6):66. https://doi.org/10.3390/hematolrep17060066
Chicago/Turabian StyleAbddaoui, Meryeme, Youssef Aghlallou, Imane Tlemçani, and Moncef Amrani Hassani. 2025. "Acute Promyelocytic Leukemia: Pathophysiology, Diagnosis and Clinical Management" Hematology Reports 17, no. 6: 66. https://doi.org/10.3390/hematolrep17060066
APA StyleAbddaoui, M., Aghlallou, Y., Tlemçani, I., & Amrani Hassani, M. (2025). Acute Promyelocytic Leukemia: Pathophysiology, Diagnosis and Clinical Management. Hematology Reports, 17(6), 66. https://doi.org/10.3390/hematolrep17060066