Hodgkin Lymphoma Associated with Common Variable Immunodeficiency: The Role of Early Diagnosis and Multidisciplinary Management
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. First Case
3.2. Second Case
4. Discussion
4.1. Diagnosis and Timing
4.2. Infection Control and the Role of EBV
4.3. Treatment Modifications
4.4. Targeted and Novel Therapies
4.5. Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| ABVD | doxorubicin, bleomycin, vinblastine, and dacarbazine |
| AVD | doxorubicin, vinblastine, and dacarbazine |
| BV | brentuximab vedotin |
| CMR | complete metabolic remission |
| CMV | Cytomegalovirus |
| COPP | cyclophosphamide, vincristine, procarbazine, and prednisolone |
| CT | computed tomography |
| CTLA4 | cytotoxic T-lymphocyte associated protein 4 |
| CVID | Common Variable Immunodeficiency |
| DHAC | dexamethasone, cytarabine, carboplatin |
| DHAP | dexamethasone, high-dose cytarabine and cisplatin |
| EBV | Epstein–Barr virus |
| EBVD | epirubicin, bleomycin, vinblastine, and dacarbazine |
| HL | Hodgkin lymphoma |
| HLH | Hemophagocytic Lymphohistiocytosis |
| ICON | International Consensus Document |
| IgA, IgG, IgM | Immunoglobulin A, G, M |
| IVIG | intravenous immunoglobulin |
| LMP1/LMP2 | latent membrane protein 1/2 |
| LRBA | LPS-responsive beige-like anchor protein |
| MOPP | mechlorethamine, vincristine, procarbazine, prednisone |
| NFKB1/2 | nuclear factor kappa-light-chain-enhancer of activated B cells 1 and 2 |
| NHL | Non-Hodgkin Lymphoma |
| PD-1 | programmed cell death protein 1 |
| PD-1-i | programmed cell death protein 1 inhibitor |
| PD-L1/D-L2 | programmed cell death-ligand 1/2 |
| PET/CT | positron emission tomography–computed tomography |
| PIK3CD | phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta |
| PMR | partial metabolic response |
| RS Cells | Reed–Sternberg Cells |
| RT | radiation therapy |
References
- Ho, H.; Cunningham-Rundles, C. Non-infectious Complications of Common Variable Immunodeficiency: Updated Clinical Spectrum, Sequelae, and Insights to Pathogenesis. Front. Immunol. 2020, 11, 149. [Google Scholar] [CrossRef]
- Fevang, B. Treatment of inflammatory complications in common variable immunodeficiency (CVID): Current concepts and future perspectives. Expert Rev. Clin. Immunol. 2023, 19, 627–638. [Google Scholar] [CrossRef]
- Tam, J.S.; Routes, J.M. Common variable immunodeficiency. Am. J. Rhinol. Allergy 2013, 27, 260–265. [Google Scholar] [CrossRef]
- Fekrvand, S.; Khanmohammadi, S.; Abolhassani, H.; Yazdani, R. B- and T-Cell Subset Abnormalities in Monogenic Common Variable Immunodeficiency. Front. Immunol. 2022, 13, 912826. [Google Scholar] [CrossRef]
- Song, J.; Lleo, A.; Yang, G.X.; Zhang, W.; Bowlus, C.L.; Gershwin, M.E.; Leung, P.S.C. Common Variable Immunodeficiency and Liver Involvement. Clin. Rev. Allerg. Immunol. 2018, 55, 340–351. [Google Scholar] [CrossRef]
- Latour, S.; Winter, S. Inherited Immunodeficiencies With High Predisposition to Epstein–Barr Virus-Driven Lymphoproliferative Diseases. Front. Immunol. 2018, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Pociupany, M.; Snoeck, R.; Dierickx, D.; Andrei, G. Treatment of Epstein-Barr Virus infection in immunocompromised patients. Biochem. Pharmacol. 2024, 225, 116270. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.-H.; Bartlett, N.L. Hodgkin lymphoma. Nat. Rev. Dis. Primers 2020, 6, 61. [Google Scholar] [CrossRef]
- Masel, R.; Roche, M.E.; Martinez-Outschoorn, U. Hodgkin Lymphoma: A disease shaped by the tumor micro- and macroenvironment. Best Pract. Res. Clin. Haematol. 2023, 36, 101514. [Google Scholar] [CrossRef]
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Friedberg, J.W. Classic Hodgkin Lymphoma in Adolescents and Young Adults. J. Clin. Oncol. 2024, 42, 653–664. [Google Scholar] [CrossRef]
- Roemer, M.G.M.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar] [CrossRef]
- Tak Manesh, A.; Azizi, G.; Heydari, A.; Kiaee, F.; Shaghaghi, M.; Hossein-Khannazer, N.; Yazdani, R.; Abolhassani, H.; Aghamohammadi, A. Epidemiology and pathophysiology of malignancy in common variable immunodeficiency? Allergol. Immunopathol. 2017, 45, 602–615. [Google Scholar] [CrossRef]
- Bednarska, K.; Chowdhury, R.; Tobin, J.W.D.; Swain, F.; Keane, C.; Boyle, S.; Khanna, R.; Gandhi, M.K. Epstein–Barr virus-associated lymphomas decoded. Br. J. Haematol. 2024, 204, 415–433. [Google Scholar] [CrossRef]
- Toner, K.; Bollard, C.M. EBV+ lymphoproliferative diseases: Opportunities for leveraging EBV as a therapeutic target. Blood 2022, 139, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Alibrahim, M.N.; Gloghini, A.; Carbone, A. Immune Deficiency/Dysregulation-Associated EBV-Positive Classic Hodgkin Lymphoma. Cancers 2025, 17, 1433. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Gereige, J.D.; Maglione, P.J. State-of-the-art diagnostic evaluation of common variable immunodeficiency. Ann. Allergy Asthma Immunol. 2021, 127, 19–27. [Google Scholar] [CrossRef]
- Kishore, E.; Gyabaah, F.; Deoker, A. Common Variable Immunodeficiency and Hodgkin Lymphoma in a 50-Year-Old Male. Cureus 2024, 16, e58989. [Google Scholar] [CrossRef]
- Ellwood, A.; Hamm, C. Common Variable Immunodeficiency Presenting with Hodgkin’s Disease. Blood 2005, 106, 4642. [Google Scholar] [CrossRef]
- Rael, E.; Rakszawski, K.; Koller, K.; Bayerl, M.; Butte, M.; Zheng, H. Treatment with rituximab and brentuximab vedotin in a patient of common variable immune deficiency-associated classic Hodgkin lymphoma. Biomark. Res. 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Ö.; Orhan, M.F.; Koçer, G.B.; Büyükavcı, M. Development of Hodgkin Lymphoma in a Patient with Common Variable Immunodeficiency. Istanb. Med. J. 2019, 20, 583–584. [Google Scholar] [CrossRef]
- Tootoonchi, P. Hodgkin Lymphoma in a Girl with Common Variable Immune Deficiency: A Case Report and Review of Literature. JCSTM 2023, 5, 000121. [Google Scholar] [CrossRef]
- Tatci, E.; Biner, I.U.; Tanyildiz, H.G.; Ozmen, O.; Gokcek, A.; Sahin, G.; Tazeler, Z. 18F-FDG PET/CT Imaging of Hodgkin Lymphoma in a Child with Common Variable Immunodeficiency. J. Nucl. Med. Technol. 2016, 44, 259–260. [Google Scholar] [CrossRef]
- Aghamohammadi, A.; Rezaei, N.; Gharagozlou, M.; Ramyar, A.; Mahjoub, F.; Rezaei-Kalantari, K.; Moin, M. Hodgkin Lymphoma in Two Siblings with Common Variable Immunodeficiency. Pediatr. Hematol. Oncol. 2007, 24, 337–342. [Google Scholar] [CrossRef]
- Gunther, J.R.; Pinnix, C.C.; Glober, G.R.; Christopherson, K.M.; Fang, P.; Lee, H.J.; Ahmed, S.; Steiner, R.E.; Nair, R.; Strati, P.; et al. Partial omission of bleomycin for early-stage Hodgkin lymphoma patients treated with combined modality therapy: Does incomplete ABVD lead to inferior outcomes? eJHaem 2020, 1, 272–276. [Google Scholar] [CrossRef]
- Yahia, A.E.; Alsakkaf, A.; Alshehri, H.; Alshehry, N.F.; Alrajhi, A.M.; Albtoosh, B.; Alhalouly, T.; Alnoamani, M.S.; Alhumaid, M.; Alzahrani, K.; et al. Bleomycin Induced Lung Toxicity in Hodgkin Lymphoma Patients Treated with ABVD Regimen- a Single Center Experience from Riyadh, Saudi Arabia. Blood 2024, 144, 6374. [Google Scholar] [CrossRef]
- Ansell, S.M.; Radford, J.; Connors, J.M.; Długosz-Danecka, M.; Kim, W.-S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.; Hutchings, M.; et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2022, 387, 310–320. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Wang, L.; Zhang, R.; Xie, J. Frontline brentuximab vedotin-based therapy for newly diagnosed classical Hodgkin lymphoma: A meta-analysis of randomized controlled trials. Front. Oncol. 2025, 15, 1636923. [Google Scholar] [CrossRef]
- Borchmann, P.; Ferdinandus, J.; Schneider, G.; Moccia, A.; Greil, R.; Hertzberg, M.; Schaub, V.; Hüttmann, A.; Keil, F.; Dierlamm, J.; et al. Assessing the efficacy and tolerability of PET-guided BrECADD versus eBEACOPP in advanced-stage, classical Hodgkin lymphoma (HD21): A randomised, multicentre, parallel, open-label, phase 3 trial. Lancet 2024, 404, 341–352. [Google Scholar] [CrossRef]
- Herrera, A.F.; LeBlanc, M.; Castellino, S.M.; Li, H.; Rutherford, S.C.; Evens, A.M.; Davison, K.; Punnett, A.; Parsons, S.K.; Ahmed, S.; et al. Nivolumab+AVD in Advanced-Stage Classic Hodgkin’s Lymphoma. N. Engl. J. Med. 2024, 391, 1379–1389. [Google Scholar] [CrossRef]
- Sun, C.; Chen, H.; Wang, Y.; Zheng, C. Safety and efficacy of PD-1 and PD-L1 inhibitors in relapsed and refractory Hodgkin’s lymphoma: A systematic review and meta-analysis of 20 prospective studies. Hematology 2023, 28, 2181749. [Google Scholar] [CrossRef]
- Paquin-Proulx, D.; Sandberg, J.K. Persistent Immune Activation in CVID and the Role of IVIg in Its Suppression. Front. Immunol. 2014, 5, 637. [Google Scholar] [CrossRef]
- Baek, D.W.; Song, G.-Y.; Lee, H.S.; Do, Y.R.; Lee, J.H.; Yhim, H.-Y.; Moon, J.H.; Yang, D.-H. Clinical efficacy of prophylactic intravenous immunoglobulin for elderly DLBCL patients with hypogammaglobulinemia in the COVID-19 pandemic era. Front. Oncol. 2024, 14, 1380492. [Google Scholar] [CrossRef]
- Cunningham-Rundles, C. How I treat common variable immune deficiency. Blood 2010, 116, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, H.S.; Ouyang, W.; Lo, B.; Deenick, E.K.; Niemela, J.E.; Avery, D.T.; Schickel, J.-N.; Tran, D.Q.; Stoddard, J.; Zhang, Y.; et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014, 345, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Fliegauf, M.; Bryant, V.L.; Frede, N.; Slade, C.; Woon, S.-T.; Lehnert, K.; Winzer, S.; Bulashevska, A.; Scerri, T.; Leung, E.; et al. Haploinsufficiency of the NF-κB1 Subunit p50 in Common Variable Immunodeficiency. Am. J. Hum. Genet. 2015, 97, 389–403. [Google Scholar] [CrossRef] [PubMed]

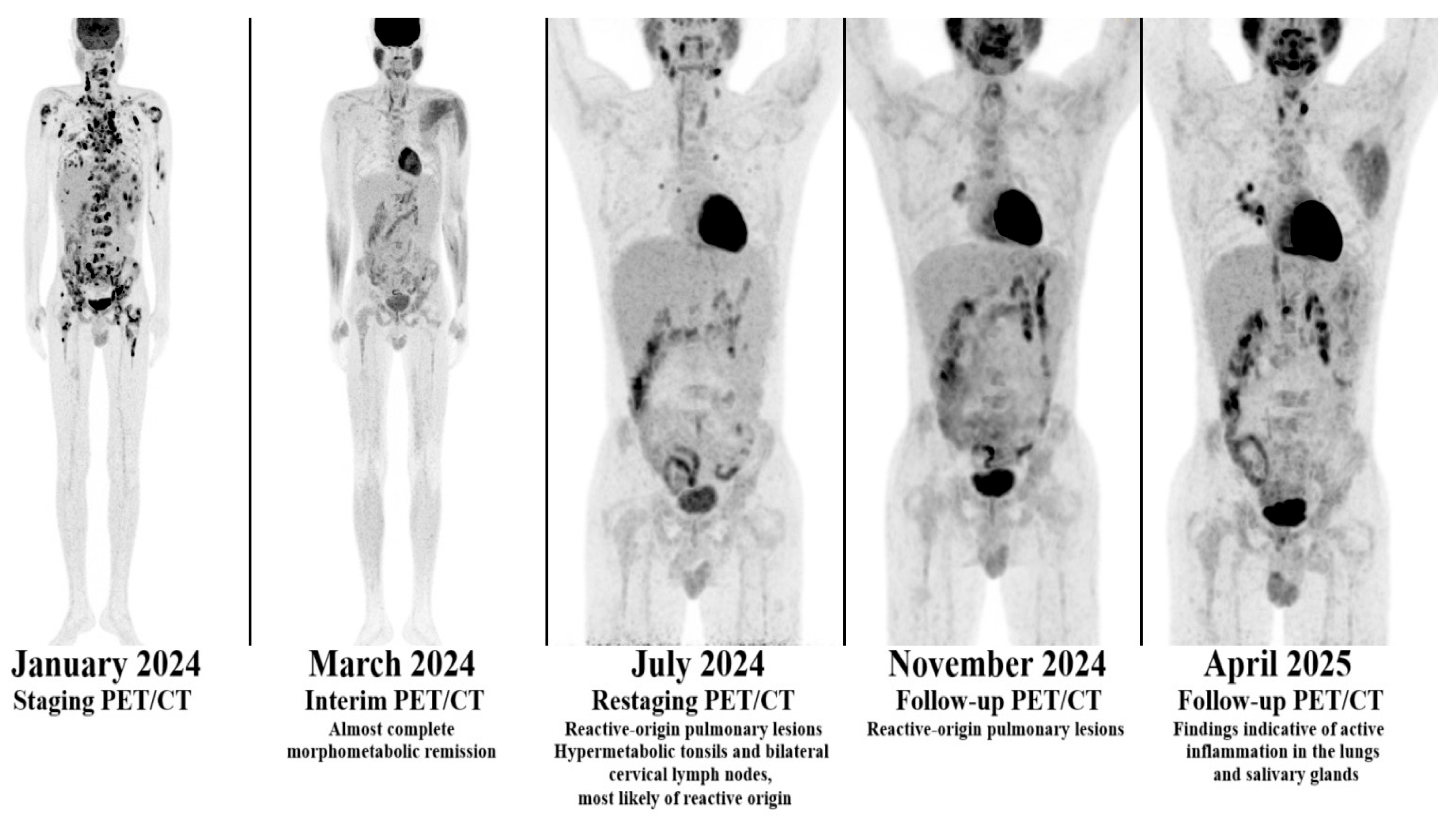
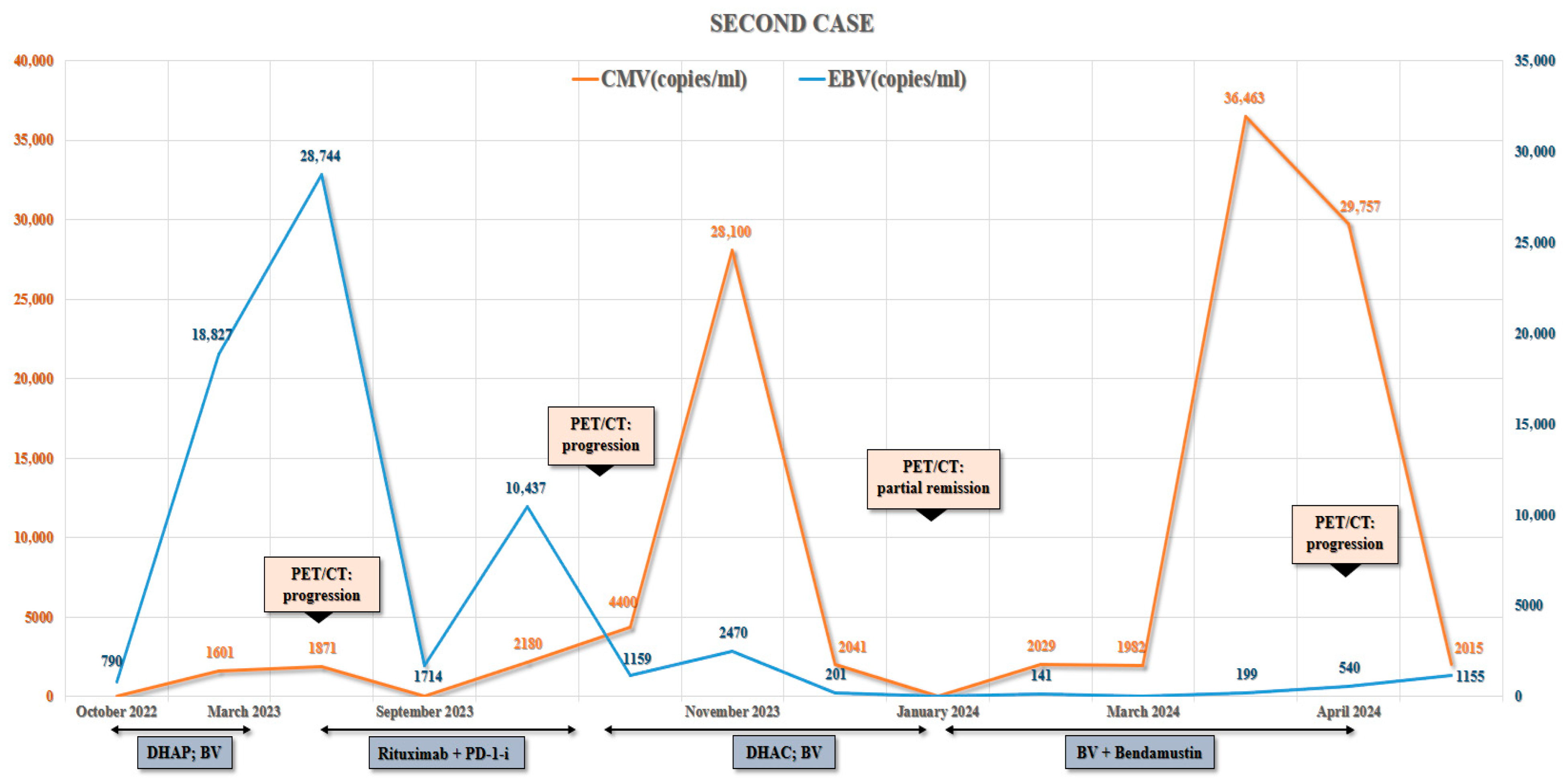
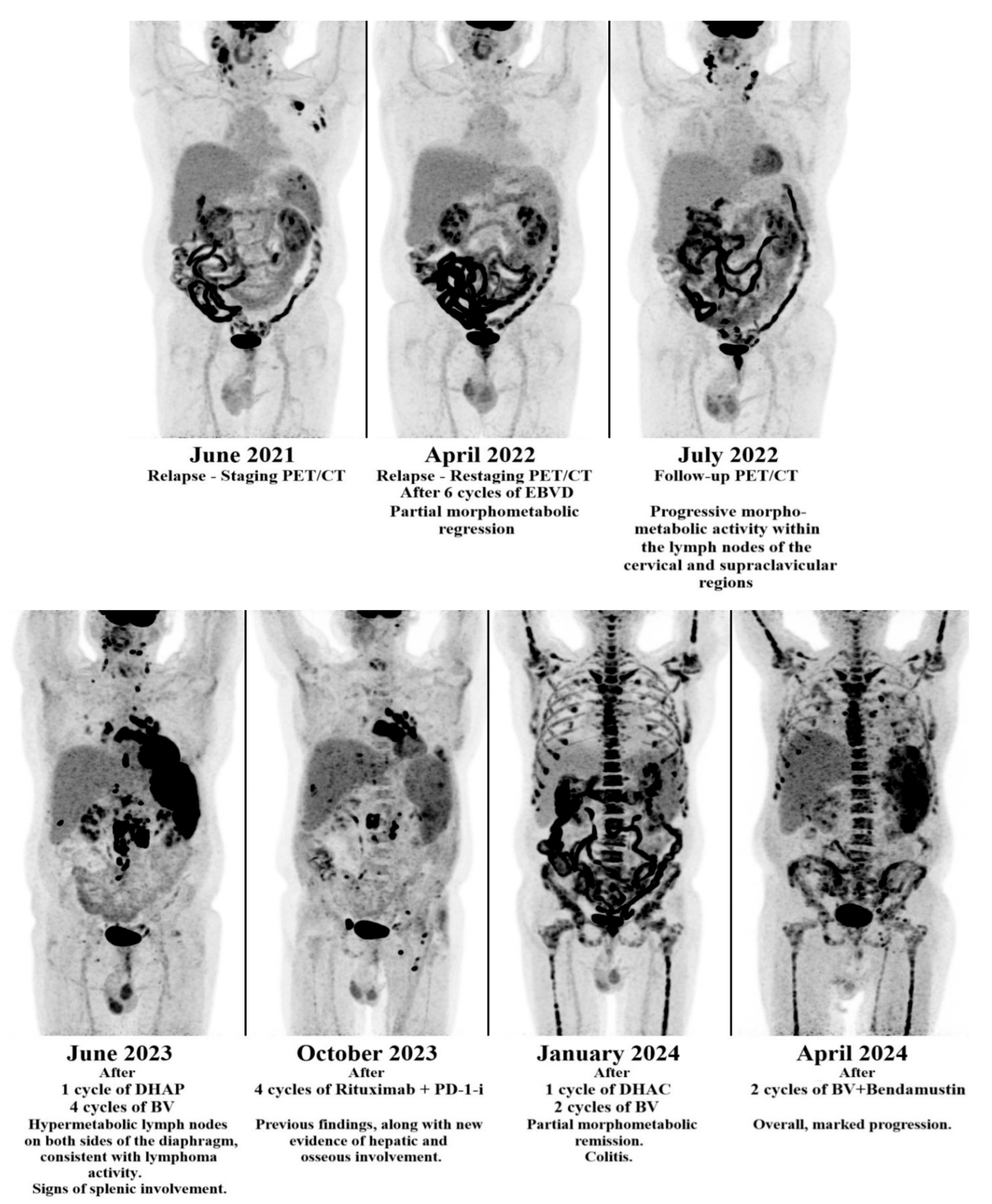
| Case | Age | Treatment | EBV Status |
|---|---|---|---|
| Case 1 (Own) | 33 | BV + AVD → CMR | Positive |
| Case 2 (Own) | 56 | EBVD → DHAP → BV →PD-1-i + Rituximab → DHAC → BV + Bendamustine → Death | Positive |
| Rael et al. [20] | 25 | Rituximab + BV → CMR | Positive |
| Kishore et al. [18] | 50 | Not specified | Not specified |
| Ellwood et al. [19] | Not specified | Not specified | Not specified |
| Özdemir et al. [21] | 9 | Reduced-dose ABVD → CMR | Negative |
| Tootoonchi et al. [22] | 13 | ABVD + COPP → CMR | Negative |
| Tatci et al. [23] | 7 | AVD + RT → Death | Not mentioned |
| Aghamohammadi et al., Case 1 [24] | 16 | MOPP + ABVD → Death | Not mentioned |
| Aghamohammadi et al., Case 2 [24] | 11 | MOPP + ABVD → Death | Not mentioned |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóthfalusi, D.; Gulyás, A.; Koncz, A.; Zöld, É.; Illés, Á.; Miltényi, Z. Hodgkin Lymphoma Associated with Common Variable Immunodeficiency: The Role of Early Diagnosis and Multidisciplinary Management. Hematol. Rep. 2025, 17, 65. https://doi.org/10.3390/hematolrep17060065
Tóthfalusi D, Gulyás A, Koncz A, Zöld É, Illés Á, Miltényi Z. Hodgkin Lymphoma Associated with Common Variable Immunodeficiency: The Role of Early Diagnosis and Multidisciplinary Management. Hematology Reports. 2025; 17(6):65. https://doi.org/10.3390/hematolrep17060065
Chicago/Turabian StyleTóthfalusi, Dávid, Anita Gulyás, Anna Koncz, Éva Zöld, Árpád Illés, and Zsófia Miltényi. 2025. "Hodgkin Lymphoma Associated with Common Variable Immunodeficiency: The Role of Early Diagnosis and Multidisciplinary Management" Hematology Reports 17, no. 6: 65. https://doi.org/10.3390/hematolrep17060065
APA StyleTóthfalusi, D., Gulyás, A., Koncz, A., Zöld, É., Illés, Á., & Miltényi, Z. (2025). Hodgkin Lymphoma Associated with Common Variable Immunodeficiency: The Role of Early Diagnosis and Multidisciplinary Management. Hematology Reports, 17(6), 65. https://doi.org/10.3390/hematolrep17060065






