Concomitant Acquired Hemophilia A and Acquired Von Willebrand Syndrome from Distinctive Autoantibodies: Case Report
Abstract
1. Introduction
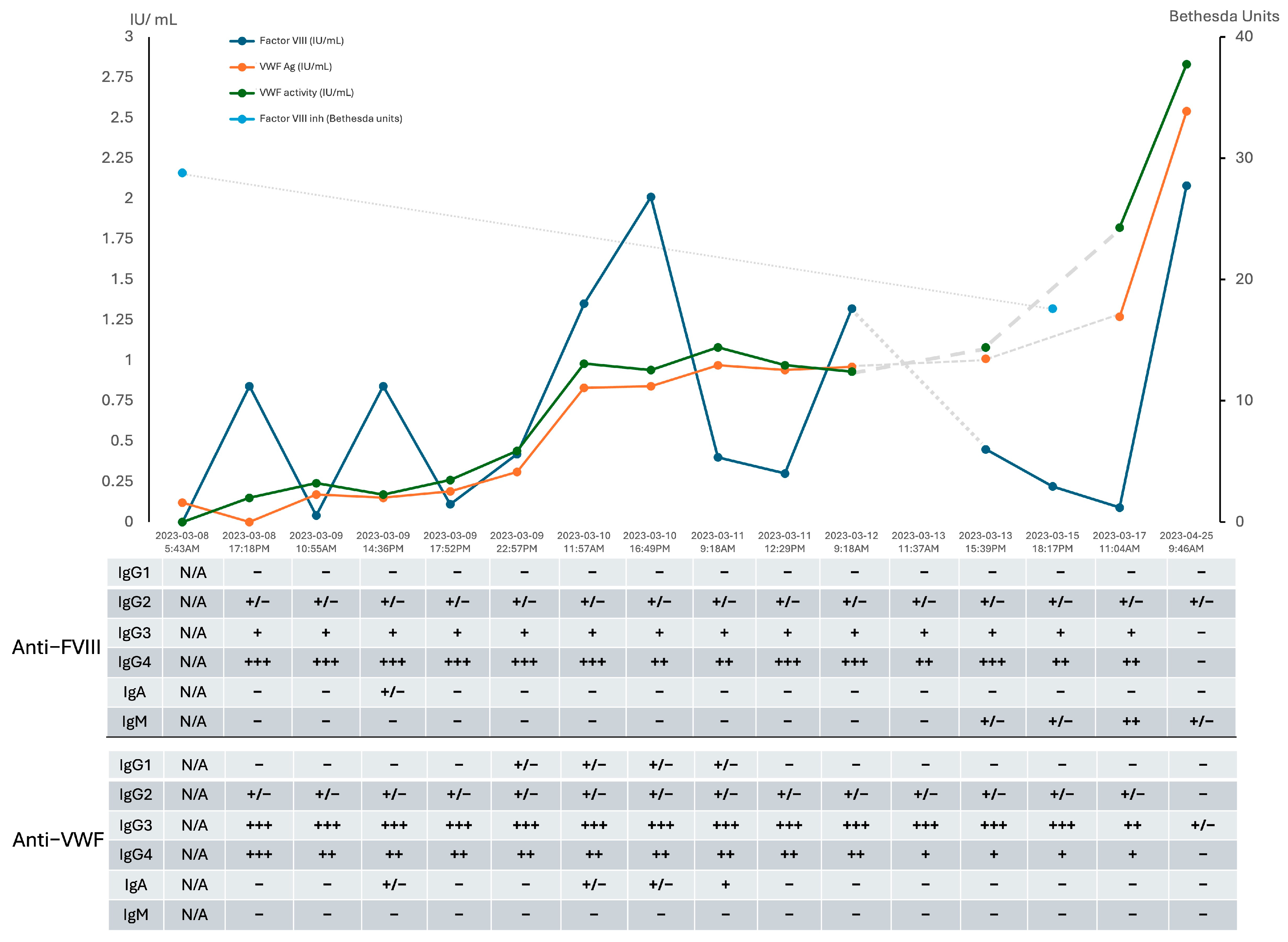
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHA | Acquired Hemophilia A |
| ANCA | Anti-Neutrophil Cytoplasmic Antibodies |
| aPTT | Activated Partial Thromboplastin Time |
| AVWS | Acquired von Willebrand Syndrome |
| BU | Bethesda Unit |
| COVID-19 | Coronavirus Disease |
| CT | Computed Tomography |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FVIII | Factor VIII |
| FIX | Factor IX |
| FXI | Factor XI |
| GPIb | Glycoprotein Ib |
| HIV | Human Immunodeficiency Virus |
| HMW | High Molecular Weight |
| IVIG | Intravenous Immunoglobulins |
| PRBC | Packed Red Blood Cells |
| STEMI | ST-elevation Myocardial Infarction |
| VWFpp | VWF Propeptide Antigen |
References
- Kruse-Jarres, R.; Kempton, C.L.; Baudo, F.; Collins, P.W.; Knoebl, P.; Leissinger, C.A.; Tiede, A.; Kessler, C.M. Acquired hemophilia A: Updated review of evidence and treatment guidance. Am. J. Hematol. 2017, 92, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Knoebl, P.; Marco, P.; Baudo, F.; Collins, P.; Huth-Kühne, A.; Nemes, L.; Pellegrini, F.; Tengborn, L.; Lévesque, H. Demographic and clinical data in acquired hemophilia A: Results from the European Acquired Haemophilia Registry (EACH2). J. Thromb. Haemost. 2012, 10, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mannucci, P.M. Alloantibodies in von Willebrand Disease. Semin. Thromb. Hemost. 2018, 44, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Athar, M.; Girgis, S.; Hassan, A.; Becker, R.C. Acquired Von Willebrand Syndrome (AVWS) in cardiovascular disease: A state of the art review for clinicians. J. Thromb. Thrombolysis 2019, 48, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mannucci, P.M. Acquired von Willebrand syndrome: Focused for hematologists. Haematologica 2020, 105, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pruthi, R.K.; Nichols, W.L. Acquired von Willebrand’s syndrome: A single institution experience. Am. J. Hematol. 2003, 72, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Dicke, C.; Holstein, K.; Schneppenheim, S.; Dittmer, R.; Schneppenheim, R.; Bokemeyer, C.; Iking-Konert, C.; Budde, U.; Langer, F. Acquired hemophilia A and von Willebrand syndrome in a patient with late-onset systemic lupus erythematosus. Exp. Hematol. Oncol. 2014, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Mansory, E.M.; Bahodi, F.; Phua, C.W. Reflex factor coagulation testing in patients with an unexplained prolonged aPTT: An institutional retrospective review. Int. J. Lab. Hematol. 2022, 44, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Santoro, R.C.; Testa, S.; Molinari, A.C.; Bernardini, S.; Golato, M.; Lippi, G.; Ageno, W.; Santagostino, E. Position paper on laboratory testing for patients with haemophilia. A consensus document from SISET, AICE, SIBioC and SIPMeL. Blood Transfus. 2019, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia panelists and co-authors. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26 (Suppl. 6), 1–158. [Google Scholar] [CrossRef]
- Castellone, D.D.; Adcock, D.M. Factor VIII Activity and Inhibitor Assays in the Diagnosis and Treatment of Hemophilia A. Semin. Thromb. Hemost. 2017, 43, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.H. Monitoring of von Willebrand factor inhibitors in patients with type 3 von Willebrand disease using a quantitative assay. Haemophilia 2021, 27, 823–829. [Google Scholar] [CrossRef] [PubMed]
- James, P.D.; Lillicrap, D.; Mannucci, P.M. Alloantibodies in von Willebrand disease. Blood 2013, 122, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.M.; Abou-Ismail, M.Y.; Lim, M.Y. Off-label use of emicizumab in persons with acquired haemophilia A and von Willebrand disease: A scoping review of the literature. Haemophilia 2022, 28, 4–17. [Google Scholar] [CrossRef] [PubMed]
| Test | Value | Units | Reference Range |
|---|---|---|---|
| Hematology | |||
| Hemoglobin | 49 | g/L | 115–160 |
| Platelets | 263 | ×109/L | 150–400 |
| Leukocytes | 6.3 | ×109/L | 4–10 |
| Coagulation | |||
| INR | 1.0 | − | 0.9–1.1 |
| aPTT | 64 | sec | 20–29 |
| FVIII Activity | <0.01 | IU/mL | 0.5–1.5 |
| FVIII Inhibitor | 28.8 | BU | <0.6 |
| FIX Activity | 1.57 | IU/mL | 0.5–2.0 |
| FXI Activity | 0.75 | IU/mL | 0.5–2.0 |
| VWF Studies | 0.5–2.0 | ||
| VWF antigen (VWF Ag) | 0.12 | IU/mL | 0.5–2.0 |
| VWF Activity (VWF:GP1bM) | <0.15 | IU/mL | 0.48–1.73 |
| Reference Laboratory Testing | |||
| VWF Propeptide (VWFpp) | 1.3 | IU/mL | 0.56–1.4 |
| VWF Activity (VWF:GP1bM) | 0.13 | IU/mL | 0.52–1.8 |
| VWF GP1bM Mixing study | Negative | − | - |
| VWF Multimers | Faint bands, no preferential loss of HMW multimers | − | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.; Bowman, M.; Bonnefoy, A.; James, P.; Phua, C.W. Concomitant Acquired Hemophilia A and Acquired Von Willebrand Syndrome from Distinctive Autoantibodies: Case Report. Hematol. Rep. 2025, 17, 52. https://doi.org/10.3390/hematolrep17050052
Yu R, Bowman M, Bonnefoy A, James P, Phua CW. Concomitant Acquired Hemophilia A and Acquired Von Willebrand Syndrome from Distinctive Autoantibodies: Case Report. Hematology Reports. 2025; 17(5):52. https://doi.org/10.3390/hematolrep17050052
Chicago/Turabian StyleYu, Richard, Mackenzie Bowman, Arnaud Bonnefoy, Paula James, and Chai W. Phua. 2025. "Concomitant Acquired Hemophilia A and Acquired Von Willebrand Syndrome from Distinctive Autoantibodies: Case Report" Hematology Reports 17, no. 5: 52. https://doi.org/10.3390/hematolrep17050052
APA StyleYu, R., Bowman, M., Bonnefoy, A., James, P., & Phua, C. W. (2025). Concomitant Acquired Hemophilia A and Acquired Von Willebrand Syndrome from Distinctive Autoantibodies: Case Report. Hematology Reports, 17(5), 52. https://doi.org/10.3390/hematolrep17050052





