Association of Cognitive Impairment with Reduced Health-Related Quality of Life and Depression Among Survivors of Thrombotic Thrombocytopenic Purpura
Abstract
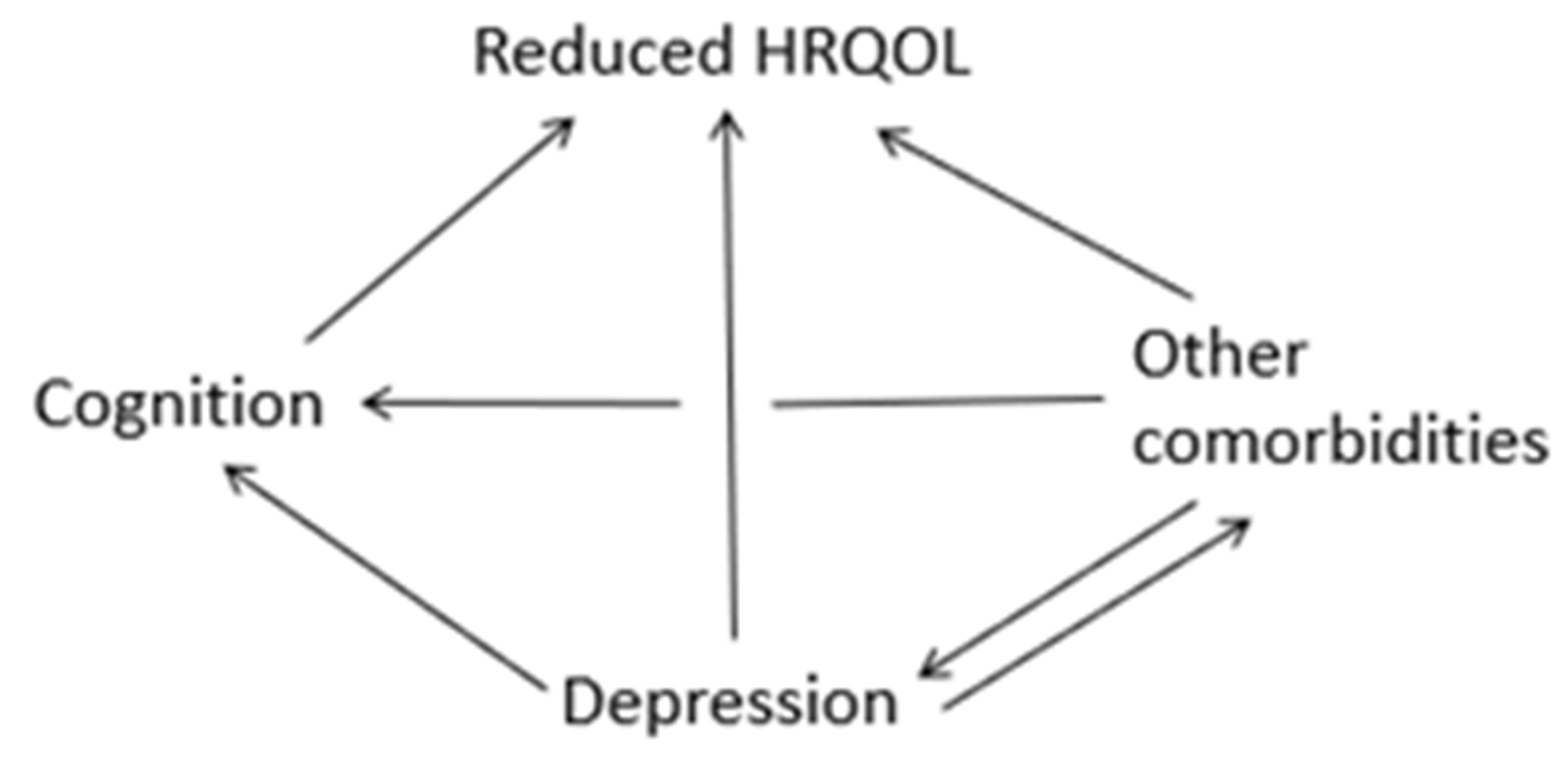
1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Data Collection Instruments
2.3. Statistical Analysis
3. Results
3.1. Study Population Characteristics
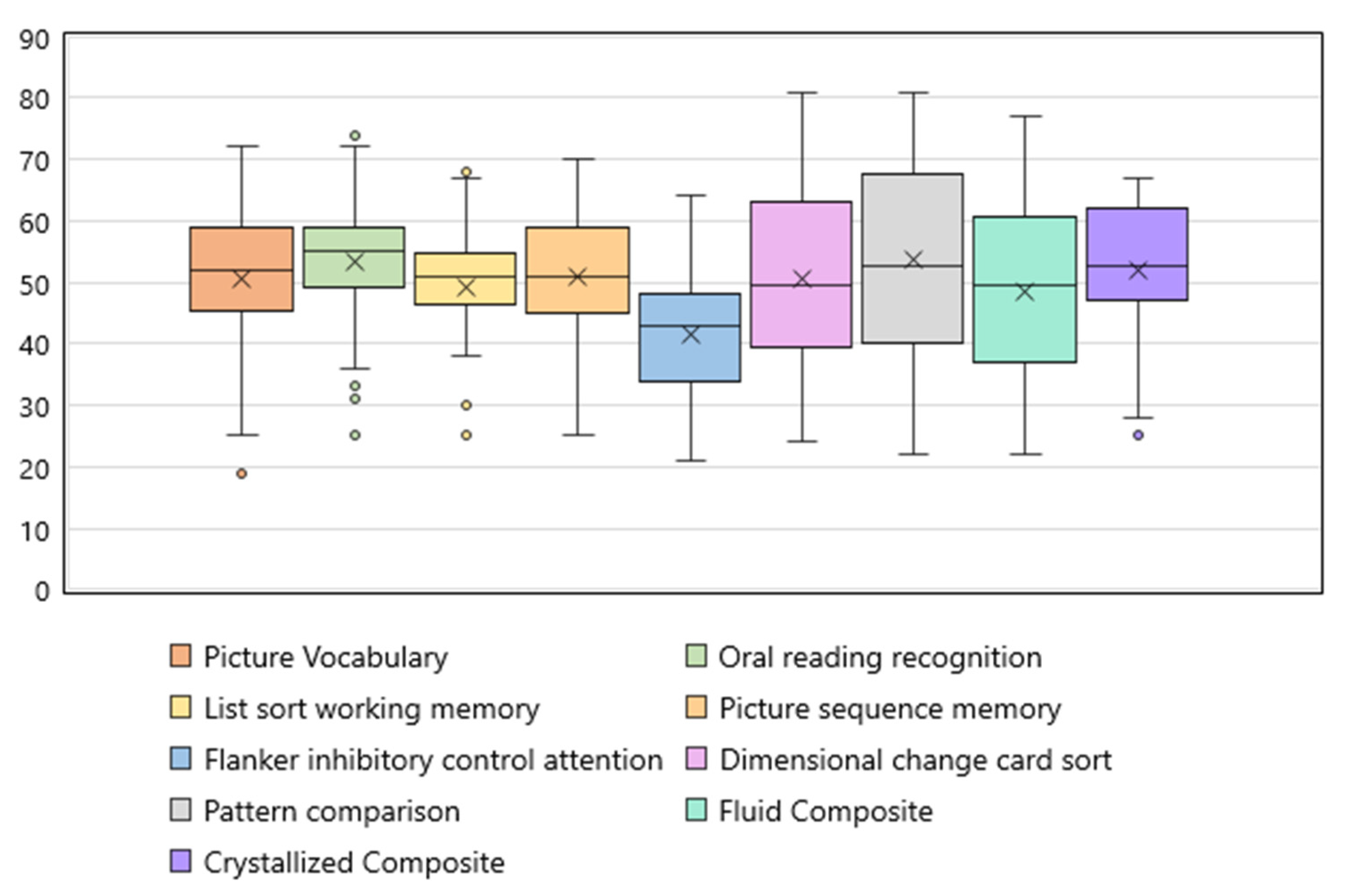
3.2. Cognitive Function in iTTP Survivors
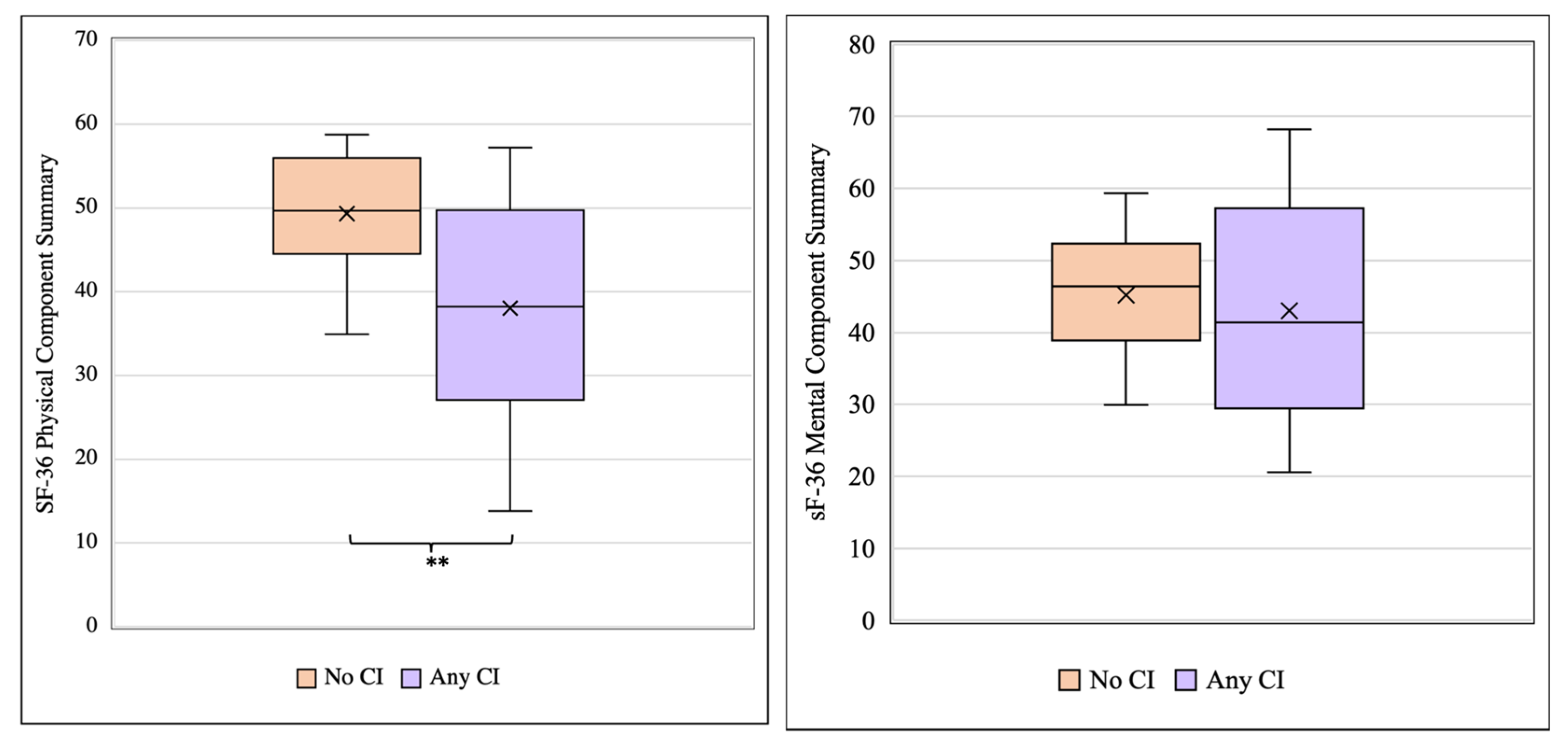
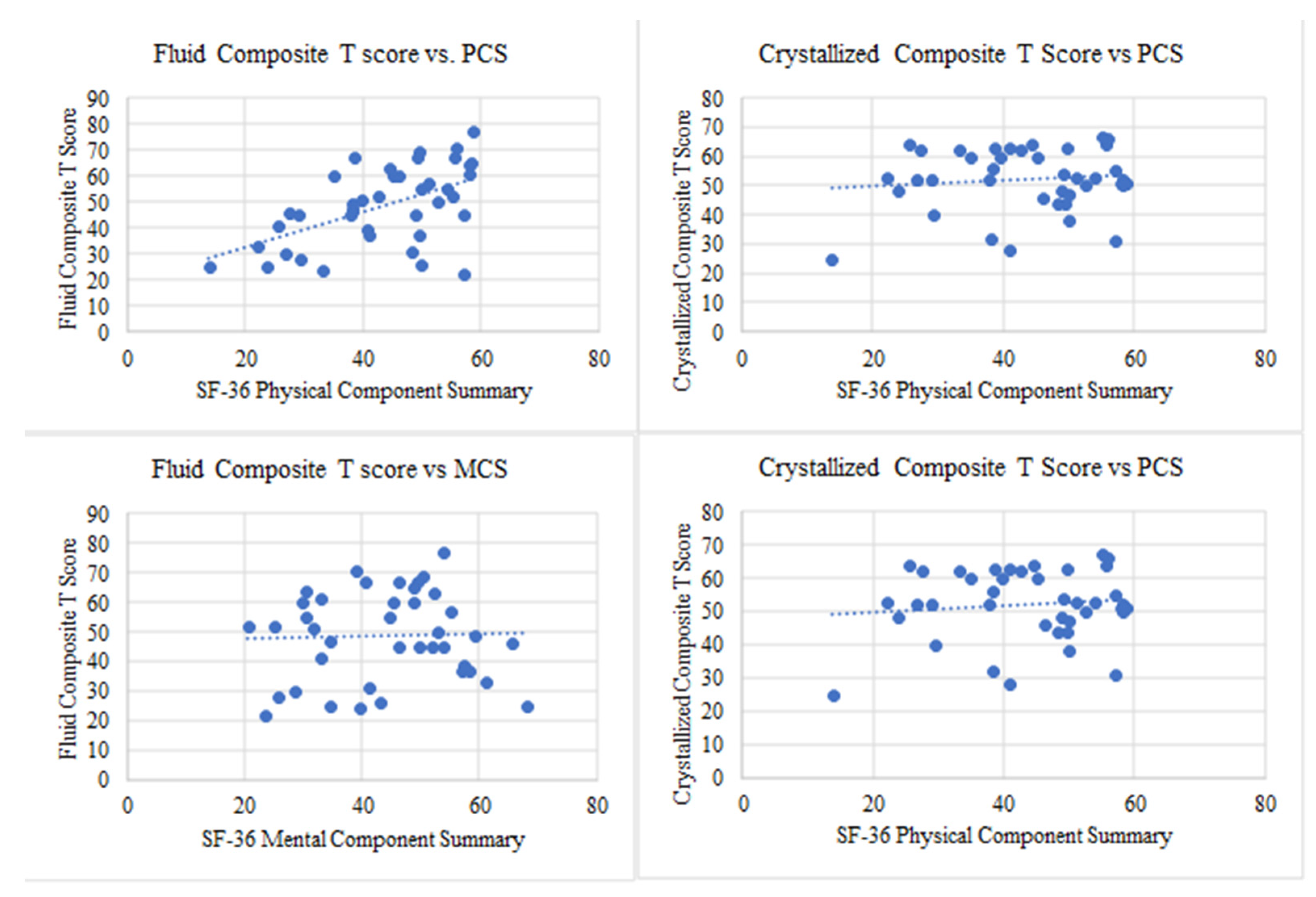
3.3. Cognitive Impairment Is Associated with Reduced Health-Related Quality of Life in TTP Survivors
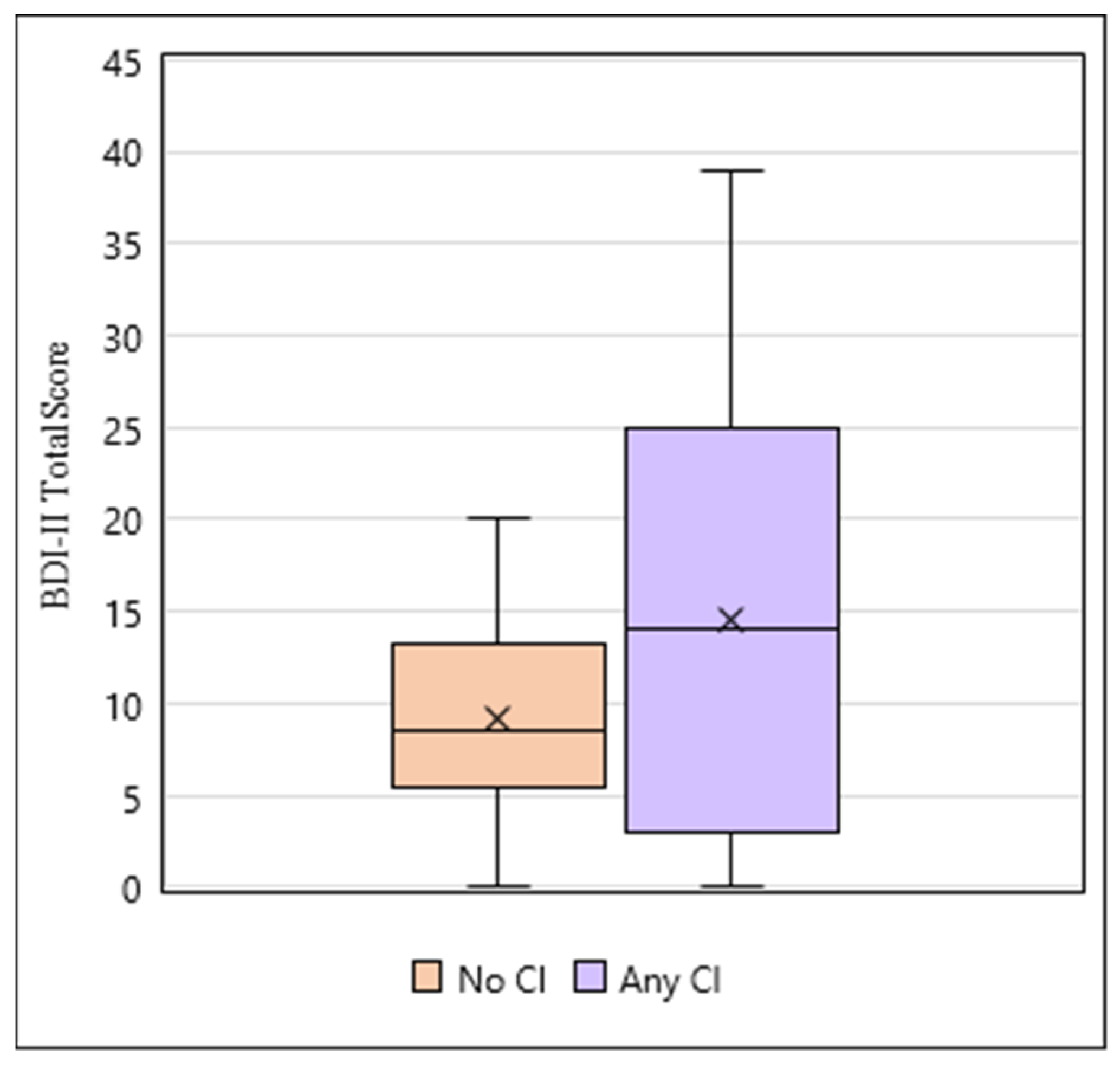
3.4. iTTP Survivors with Cognitive Impairment Have an Increase in Depressive Symptoms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Peyvandi, F.; Scully, M.; Hovinga, J.A.K.; Cataland, S.; Knöbl, P.; Wu, H.; Artoni, A.; Westwood, J.-P.; Taleghani, M.M.; Jilma, B.; et al. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2016, 374, 511–522. [Google Scholar] [CrossRef]
- Page, E.E.; Hovinga, J.A.K.; Terrell, D.R.; Vesely, S.K.; George, J.N. Thrombotic thrombocytopenic purpura: Diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv. 2017, 1, 590–600. [Google Scholar] [CrossRef]
- Deford, C.C.; Reese, J.A.; Schwartz, L.H.; Perdue, J.J.; Hovinga, J.A.K.; Lämmle, B.; Terrell, D.R.; Vesely, S.K.; George, J.N. Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood 2013, 122, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- George, J.N. TTP: Long-term outcomes following recovery. Am. Soc. Hematol. Educ. Program 2018, 2018, 548–552. [Google Scholar] [CrossRef]
- Upreti, H.; Kasmani, J.; Dane, K.; Braunstein, E.M.; Streiff, M.B.; Shanbhag, S.; Moliterno, A.R.; Sperati, C.J.; Gottesman, R.F.; Brodsky, R.A.; et al. Reduced ADAMTS13 activity during TTP remission is associated with stroke in TTP survivors. Blood 2019, 134, 1037–1045. [Google Scholar] [CrossRef]
- Tsai, H.M. The kidney in thrombotic thrombocytopenic purpura. Minerva Med. 2007, 98, 731–747. [Google Scholar] [PubMed]
- Roriz, M.; Landais, M.; Desprez, J.; Barbet, C.; Azoulay, E.; Galicier, L.; Wynckel, A.; Baudel, J.-L.; Provôt, F.; Pène, F.; et al. Risk Factors for Autoimmune Diseases Development After Thrombotic Thrombocytopenic Purpura. Medicine 2015, 94, e1598. [Google Scholar] [CrossRef]
- Lewis, Q.F.; Lanneau, M.S.; Mathias, S.D.; Terrell, D.R.; Vesely, S.K.; George, J.N. Long-term deficits in health-related quality of life after recovery from thrombotic thrombocytopenic purpura. Transfusion 2009, 49, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.S.; Lewis, Q.F.; Scott, J.G.; Hovinga, J.A.K.; Lämmle, B.; Terrell, D.R.; Vesely, S.K.; George, J.N. Cognitive deficits after recovery from thrombotic thrombocytopenic purpura. Transfusion 2009, 49, 1092–1101. [Google Scholar] [CrossRef]
- Falter, T.; Schmitt, V.; Herold, S.; Weyer, V.; von Auer, C.; Wagner, S.; Hefner, G.; Beutel, M.; Lackner, K.; Lämmle, B.; et al. Depression and cognitive deficits as long-term consequences of thrombotic thrombocytopenic purpura. Transfusion 2017, 57, 1152–1162. [Google Scholar] [CrossRef]
- Han, B.; Page, E.E.; Stewart, L.M.; Deford, C.C.; Scott, J.G.; Schwartz, L.H.; Perdue, J.J.; Terrell, D.R.; Vesely, S.K.; George, J.N. Depression and cognitive impairment following recovery from thrombotic thrombocytopenic purpura. Am. J. Hematol. 2015, 90, 709–714. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Oluwole, O.; Cataland, S.; McCrae, K.R. Post-traumatic stress disorder and depression in survivors of thrombotic thrombocytopenic purpura. Thromb. Res. 2017, 151, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Voros, V.; Fekete, S.; Tenyi, T.; Rihmer, Z.; Szili, I.; Osvath, P. Untreated depressive symptoms significantly worsen quality of life in old age and may lead to the misdiagnosis of dementia: A cross-sectional study. Ann. Gen. Psychiatry 2020, 19, 52. [Google Scholar] [CrossRef]
- Williams, A.M.; Lindholm, J.; Cook, D.; Siddiqui, F.; Ghanem, T.A.; Chang, S.S. Association Between Cognitive Function and Quality of Life in Patients With Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Stites, S.D.; Harkins, K.; Rubright, J.D.; Karlawish, J. Relationships Between Cognitive Complaints and Quality of Life in Older Adults With Mild Cognitive Impairment, Mild Alzheimer Disease Dementia, and Normal Cognition. Alzheimer Dis. Assoc. Disord. 2018, 32, 276–283. [Google Scholar] [CrossRef]
- Knight, M.J.; Lyrtzis, E.; Baune, B.T. The association of cognitive deficits with mental and physical Quality of Life in Major Depressive Disorder. Compr. Psychiatry 2020, 97, 152147. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Slotkin, J.; Carlozzi, N.E.; Bauer, P.J.; Wallner-Allen, K.; Fox, N.; et al. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: Validation in an adult sample. J. Int. Neuropsychol. Soc. 2014, 20, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Gorenstein, C. Assessment of depression in medical patients: A systematic review of the utility of the Beck Depression Inventory-II. Clinics 2013, 68, 1274–1287. [Google Scholar] [CrossRef]
- Ware, J.; Snoww, K.; Ma, K.; Bg, G. SF36 Health Survey: Manual and Interpretation Guide; Quality Metric, Inc.: Johnston, RI, USA, 1993; Volume 1993, p. 30. [Google Scholar]
- Cataland, S.R.; Scully, M.A.; Paskavitz, J.; Maruff, P.; Witkoff, L.; Jin, M.; Uva, N.; Gilbert, J.C.; Wu, H.M. Evidence of persistent neurologic injury following thrombotic thrombocytopenic purpura. Am. J. Hematol. 2011, 86, 87–89. [Google Scholar] [CrossRef]
- Yu, J.; Wei, A.; Pan, X.-Z.; Gerber, G.F.; Hussain, S.; Moliterno, A.R.; Streiff, M.B.; Kraus, P.; Logue, C.; Yui, J.; et al. Silent Cerebral Infarction on Brain MRI Is Associated with Cognitive Impairment in Ittp Survivors in Hematological Remission. Blood 2021, 138 (Suppl. 1), 774. [Google Scholar] [CrossRef]
- Onandia-Hinchado, I.; Diaz-Orueta, U. Health Related Quality of Life in Individuals with Cognitive Decline and Discrepancies Between Patients and Their Proxies. Arch. Gerontol. Geriatr. 2019, 85, 103914. [Google Scholar]
- Daly, M.; Sutin, A.R.; Robinson, E. Depression reported by US adults in 2017–2018 and March and April 2020. J. Affect. Disord. 2021, 278, 131–135. [Google Scholar] [CrossRef]
- Carey, M.; Small, H.; Yoong, S.L.; Boyes, A.; Bisquera, A.; Sanson-Fisher, R. Prevalence of comorbid depression and obesity in general practice: A cross-sectional survey. Br. J. Gen. Pract. 2014, 64, e122–e127. [Google Scholar] [CrossRef]
- Ettman, C.K.; Abdalla, S.M.; Cohen, G.H.; Sampson, L.; Vivier, P.M.; Galea, S. Prevalence of Depression Symptoms in US Adults Before and During the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2019686. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Qiu, V.; Cederlund, A.; Borg, A.; Lindblom, J.; Emamikia, S.; Enman, Y.; Lampa, J.; Parodis, I. Adverse Health-Related Quality of Life Outcome Despite Adequate Clinical Response to Treatment in Systemic Lupus Erythematosus. Front. Med. 2021, 8, 651249. [Google Scholar] [CrossRef]
- Radin, M.; El Hasbani, G.; Barinotti, A.; Roccatello, D.; Uthman, I.; Taher, A.; Sciascia, S. Quality of life measures in Systemic Lupus Erythematosus: A systematic review. Reumatismo 2022, 73, 208–228. [Google Scholar] [CrossRef]
- Xie, J.; Wu, E.Q.; Zheng, Z.-J.; Sullivan, P.W.; Zhan, L.; Labarthe, D.R. Patient-reported health status in coronary heart disease in the United States: Age, sex, racial, and ethnic differences. Circulation 2008, 118, 491–497. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Van Exel, E.; Jacobs, J.; Korswagen, L.-A.; Voskuyl, A.; Stek, M.; Dekker, J.; Bultink, I. Depression in systemic lupus erythematosus, dependent on or independent of severity of disease. Lupus 2013, 22, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | |
|---|---|
| Age, years (median, IQR) | 51 (39, 60) |
| Female sex (%) | 76.6 |
| Race (%) | |
| Black/African American | 76.6 |
| White | 16.1 |
| Other | 4.3 |
| No. of TTP episodes (median, IQR) | 2 (1, 3.5) |
| Comorbidities (%) | |
| Hypertension | 42.6 |
| Stroke | 19.1 |
| Chronic Kidney Disease | 8.5 |
| Diabetes Mellitus II | 14.9 |
| Obesity (BMI > 30) | 23.4 |
| Systemic Lupus Erythematosus | 19.1 |
| Hyperlipidemia | 19.1 |
| Psychiatric Diagnoses (%) | |
| PTSD | 6.4 |
| Depression | 31.9 |
| Anxiety | 21.3 |
| SF-36 Variable * | TTP Survivors (N = 47) | General Population (N = 2474) | p | |
|---|---|---|---|---|
| Physical Components | Physical Functioning | 68.6 ± 27.8 | 84.2 ± 23.3 | <0.0001 |
| Role-limitation Physical | 50.5 ± 41.9 | 80.9 ± 34.0 | <0.0001 | |
| Bodily Pain | 70.2 ± 27.5 | 75.2 ± 23.7 | 0.1532 | |
| General Health | 50.2 ± 22.0 | 71.9 ± 20.3 | <0.0001 | |
| Mental Components | Vitality | 46.3 ± 23.0 | 60.9 ± 20.9 | <0.0001 |
| Social Functioning | 69.4 ± 26.4 | 83.3 ± 22.7 | <0.0001 | |
| Role-limitation Emotional | 52.2 ± 40.2 | 81.3 ± 33.0 | <0.0001 | |
| Mental Health | 65.3 ± 19.3 | 74.7 ± 18.1 | 0.0004 | |
| Summary Scores | Physical Component Summary | 43.2 ± 11.7 | 50 ± 10.0 | <0.0001 |
| Mental Component Summary | 43.2 ± 12.5 | 50 ± 10.0 | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvakumar, S.; Yu, J.; Meade, J.; Chaturvedi, S. Association of Cognitive Impairment with Reduced Health-Related Quality of Life and Depression Among Survivors of Thrombotic Thrombocytopenic Purpura. Hematol. Rep. 2025, 17, 51. https://doi.org/10.3390/hematolrep17050051
Selvakumar S, Yu J, Meade J, Chaturvedi S. Association of Cognitive Impairment with Reduced Health-Related Quality of Life and Depression Among Survivors of Thrombotic Thrombocytopenic Purpura. Hematology Reports. 2025; 17(5):51. https://doi.org/10.3390/hematolrep17050051
Chicago/Turabian StyleSelvakumar, Sruthi, Jia Yu, Jacob Meade, and Shruti Chaturvedi. 2025. "Association of Cognitive Impairment with Reduced Health-Related Quality of Life and Depression Among Survivors of Thrombotic Thrombocytopenic Purpura" Hematology Reports 17, no. 5: 51. https://doi.org/10.3390/hematolrep17050051
APA StyleSelvakumar, S., Yu, J., Meade, J., & Chaturvedi, S. (2025). Association of Cognitive Impairment with Reduced Health-Related Quality of Life and Depression Among Survivors of Thrombotic Thrombocytopenic Purpura. Hematology Reports, 17(5), 51. https://doi.org/10.3390/hematolrep17050051





