Tyrosine Kinase Inhibitor Treatment of a Patient with Chronic Myeloid Leukemia and Congenital Thrombophilia
Abstract
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valent, P.; Hadzijusufovic, E.; Schernthaner, G.H.; Wolf, D.; Rea, D.; le Coutre, P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood 2015, 125, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kantarjian, H.; Boddu, P.C.; Nogueras-González, G.M.; Verstovsek, S.; Garcia-Manero, G.; Borthakur, G.; Sasaki, K.; Kadia, T.M.; Sam, P.; et al. Analysis of cardiovascular and arteriothrombotic adverse events in chronic-phase CML patients after frontline TKIs. Blood Adv. 2019, 3, 851–861. [Google Scholar] [CrossRef]
- Martinelli, I.; De Stefano, V.; Mannucci, P.M. Inherited risk factors for venous thromboembolism. Nat. Rev. Cardiol. 2014, 11, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Pasvolsky, O.; Leader, A.; Iakobishvili, Z.; Wasserstrum, Y.; Kornowski, R.; Raanani, P. Tyrosine kinase inhibitor associated vascular toxicity in chronic myeloid leukemia. Cardiooncology 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, M.; Baccarani, M.; Saussele, S.; Guilhot, J.; Cervantes, F.; Ossenkoppele, G.; Hoffmann, V.S.; Castagnetti, F.; Hasford, J.; Hehlmann, R.; et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016, 30, 48–56. [Google Scholar] [CrossRef]
- Pintao, M.C.; Ribeiro, D.D.; Bezemer, I.D.; Garcia, A.A.; de Visser, M.C.H.; Doggen, C.J.M.; Lijfering, W.M.; Reitsma, P.H.; Rosendaal, F.R. Protein S levels and the risk of venous thrombosis: Results from the MEGA case-control study. Blood 2013, 122, 3210–3219. [Google Scholar] [CrossRef]
- García de Frutos, P.; Fuentes-Prior, P.; Hurtado, B.; Sala, N. Molecular basis of protein S deficiency. Thromb. Haemost. 2007, 98, 543–556. [Google Scholar] [CrossRef]
- Marlar, R.A.; Gausman, J.N.; Tsuda, H.; Rollins-Raval, M.A.; Brinkman, H.J.M. Recommendations for clinical laboratory testing for protein S deficiency: Communication from the SSC committee plasma coagulation inhibitors of the ISTH. J. Thromb. Haemost. 2021, 19, 68–74. [Google Scholar] [CrossRef]
- Di Minno, M.N.D.; Ambrosino, P.; Ageno, W.; Rosendaal, F.; Di Minno, G.; Dentali, F. Natural anticoagulants deficiency and the risk of venous thromboembolism: A meta-analysis of observational studies. Thromb. Res. 2015, 135, 923–932. [Google Scholar] [CrossRef]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: Pathophysiology, mechanisms and clinical management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef]
- Lijfering, W.M.; Mulder, R.; ten Kate, M.K.; Veeger, N.J.; Mulder, A.B.; van der Meer, J. Clinical relevance of decreased free protein S levels: Results from a retrospective family cohort study involving 1143 relatives. Blood 2009, 113, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Vossen, C.Y.; Walker, I.D.; Svensson, P.; Souto, J.C.; Scharrer, I.; Preston, F.E.; Palareti, G.; Pabinger, I.; van der Meer, F.J.; Makris, M.; et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1992–1997. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lamore, S.D.; Kohnken, R.A.; Peters, M.F.; Kolaja, K.L. Cardiovascular Toxicity Induced by Kinase Inhibitors: Mechanisms and Preclinical Approaches. Chem. Res. Toxicol. 2020, 33, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Wylie, A.A.; Schoepfer, J.; Jahnke, W.; Cowan-Jacob, S.W.; Loo, A.; Furet, P.; Marzinzik, A.L.; Pelle, X.; Donovan, J.; Zhu, W.; et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature 2017, 543, 733–737. [Google Scholar] [CrossRef]
- Mauro, M.J.; Hughes, T.P.; Kim, D.W.; Rea, D.; Cortes, J.E.; Hochhaus, A.; Sasaki, K.; Breccia, M.; Talpaz, M.; Ottmann, O.; et al. Asciminib monotherapy in patients with CML-CP without BCR: ABL1 T315I mutations treated with at least two prior TKIs: 4-year phase 1 safety and efficacy results. Leukemia 2023, 37, 1048–1059. [Google Scholar] [CrossRef]
- Pérez-Lamas, L.; Luna, A.; Boque, C.; Xicoy, B.; Giraldo, P.; López, R.P.; Nuño, C.R.; Heras, N.D.L.; Casterá, E.M.; Marín, J.L.; et al. Toxicity of Asciminib in Real Clinical Practice: Analysis of Side Effects and Cross-Toxicity with Tyrosine Kinase Inhibitors. Cancers 2023, 15, 1045. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Hoch, M.; Huth, F.; Manley, P.W.; Loisios-Konstantinidis, I.; Combes, F.P.; Li, Y.F.; Fu, Y.; Sy, S.K.B.; Obourn, V.; Chakraborty, A.; et al. Clinical Pharmacology of Asciminib: A Review. Clin. Pharmacokinet. 2024, 63, 1513–1528. [Google Scholar] [CrossRef]
- European Medicines Agency. Scemblix: EPAR—Product Information; EMA: Amsterdam, The Netherlands, 2022; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/scemblix (accessed on 8 September 2025).
- Tran, P.; Hanna, I.; Eggimann, F.K.; Schoepfer, J.; Ray, T.; Zhu, B.; Wang, L.; Priess, P.; Tian, X.; Hourcade-Potelleret, F.; et al. Disposition of asciminib, a potent BCR-ABL1 tyrosine kinase inhibitor, in healthy male subjects. Xenobiotica 2020, 50, 150–169. [Google Scholar] [CrossRef]
- Hoch, M.; Sengupta, T.; Hourcade-Potelleret, F. Pharmacokinetic drug interactions of asciminib with the sensitive cytochrome P450 probe substrates midazolam, warfarin, and repaglinide in healthy participants. Clin. Transl. Sci. 2022, 15, 1406–1416. [Google Scholar] [CrossRef]
- Réa, D.; Mauro, M.J.; Boquimpani, C.; Minami, Y.; Lomaia, E.; Voloshin, S.; Turkina, A.G.; Apperley, J.F.; Abdo, A.; Fogliatto, L.M.; et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood 2021, 138, 2031–2041. [Google Scholar] [CrossRef]
- Gameil, A.; Ghasoub, R.; Jafari, N.; Shafei, L.; Benkhadra, M.; Laws, S.; Fernyhough, L. Evaluating the Safety of Combining Tyrosine Kinase Inhibitors with Anticoagulants in Chronic Myeloid Leukemia: A Systematic Review. Blood 2024, 144 (Suppl. S1), 6592. [Google Scholar] [CrossRef]
- Lyon, A.R.; DFent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; García-Gutiérrez, V.; Hochhaus, A.; Kim, D.-W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef]
- Moran, J.; Bauer, K.A. Managing thromboembolic risk in patients with hereditary and acquired thrombophilias. Blood 2020, 135, 344–350. [Google Scholar] [CrossRef]
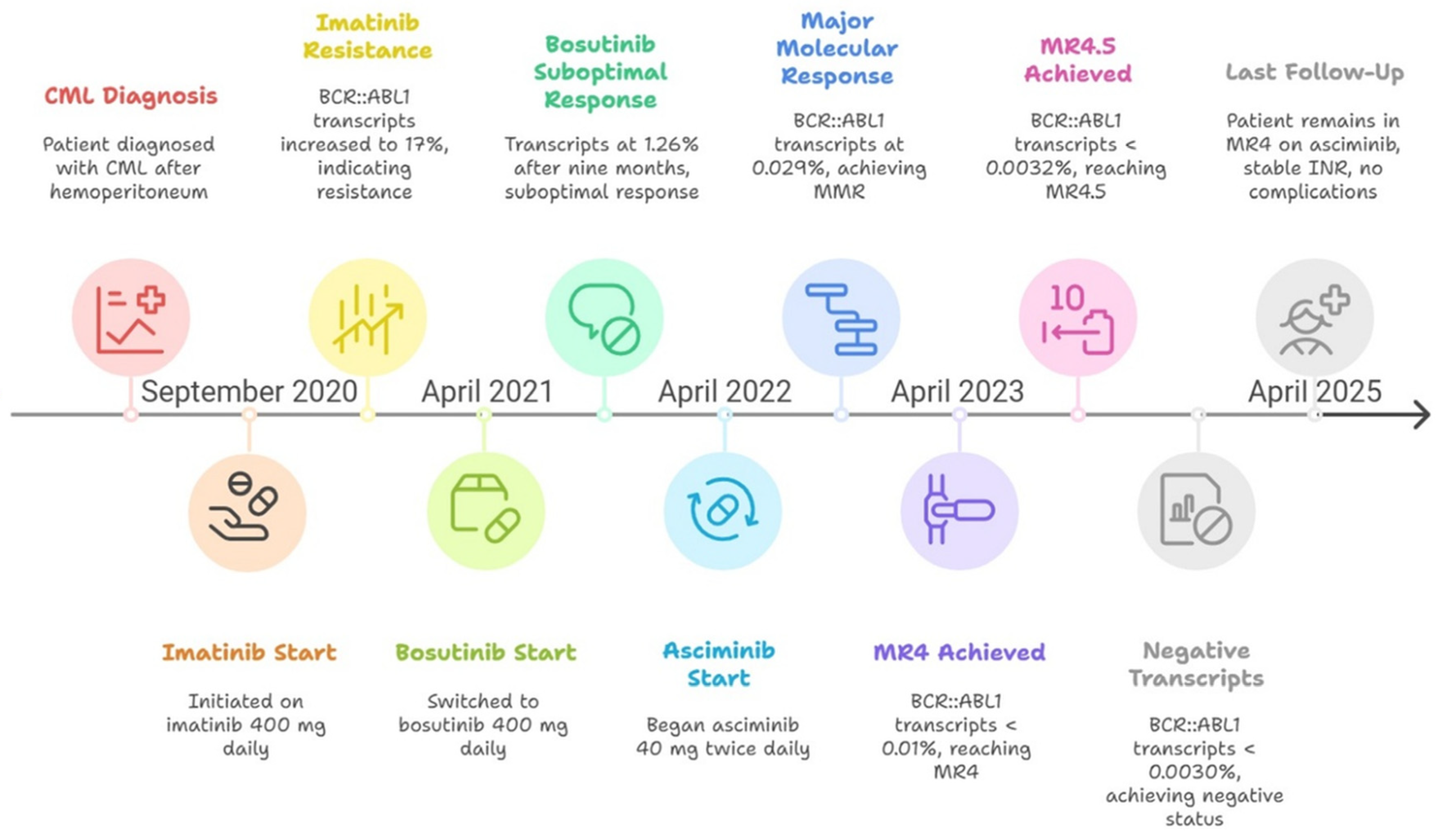
| Disease Evolution | Inhibitor | Date | BCR::ABL1/ABL1IS |
|---|---|---|---|
| Diagnosis | 20 September 2020 | 74% | |
| First-line therapy | Imatinib | 23 September 2020 | 74% |
| 20 November 2020 | 31% | ||
| 08 January 2020 | 11% | ||
| 18 February 2021 | 5.61% | ||
| 24 April 2021 | 17% | ||
| Second-line therapy | Bosutinib | 03 September 2021 | 3.77% |
| 11 October 2021 | 3.61% | ||
| 25 November 2021 | 1.69% | ||
| 05 January 2022 | 0.7% | ||
| 03 March 2022 | 1.26% | ||
| Third-line therapy | Asciminib | 06 July 2022 | 0.049% |
| 10 August 2022 | 0.029% | ||
| 10 January 2023 | 0.017% | ||
| 21 April 2023 | 0.016% | ||
| 10 August 2023 | 0.012% | ||
| 07 November 2023 | 0.0015% | ||
| 04 February 2024 | 0.0096% | ||
| 31 October 2024 | 0.0044% | ||
| 23 January 2025 | Negative * | ||
| 15 April 2025 | 0.0031% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Hernández, C.; Segura-Diaz, A.; Stuckey, R.; López-Rodríguez, J.F.; Gómez-Casares, M.T. Tyrosine Kinase Inhibitor Treatment of a Patient with Chronic Myeloid Leukemia and Congenital Thrombophilia. Hematol. Rep. 2025, 17, 47. https://doi.org/10.3390/hematolrep17050047
Herrera-Hernández C, Segura-Diaz A, Stuckey R, López-Rodríguez JF, Gómez-Casares MT. Tyrosine Kinase Inhibitor Treatment of a Patient with Chronic Myeloid Leukemia and Congenital Thrombophilia. Hematology Reports. 2025; 17(5):47. https://doi.org/10.3390/hematolrep17050047
Chicago/Turabian StyleHerrera-Hernández, Carol, Adrián Segura-Diaz, Ruth Stuckey, Juan Francisco López-Rodríguez, and María Teresa Gómez-Casares. 2025. "Tyrosine Kinase Inhibitor Treatment of a Patient with Chronic Myeloid Leukemia and Congenital Thrombophilia" Hematology Reports 17, no. 5: 47. https://doi.org/10.3390/hematolrep17050047
APA StyleHerrera-Hernández, C., Segura-Diaz, A., Stuckey, R., López-Rodríguez, J. F., & Gómez-Casares, M. T. (2025). Tyrosine Kinase Inhibitor Treatment of a Patient with Chronic Myeloid Leukemia and Congenital Thrombophilia. Hematology Reports, 17(5), 47. https://doi.org/10.3390/hematolrep17050047






