Prognostic Factors for 28-Day Mortality in Pediatric Patients with Acute Leukemia and Candidemia Following Intensive Chemotherapy: A Retrospective Study
Abstract
1. Introduction
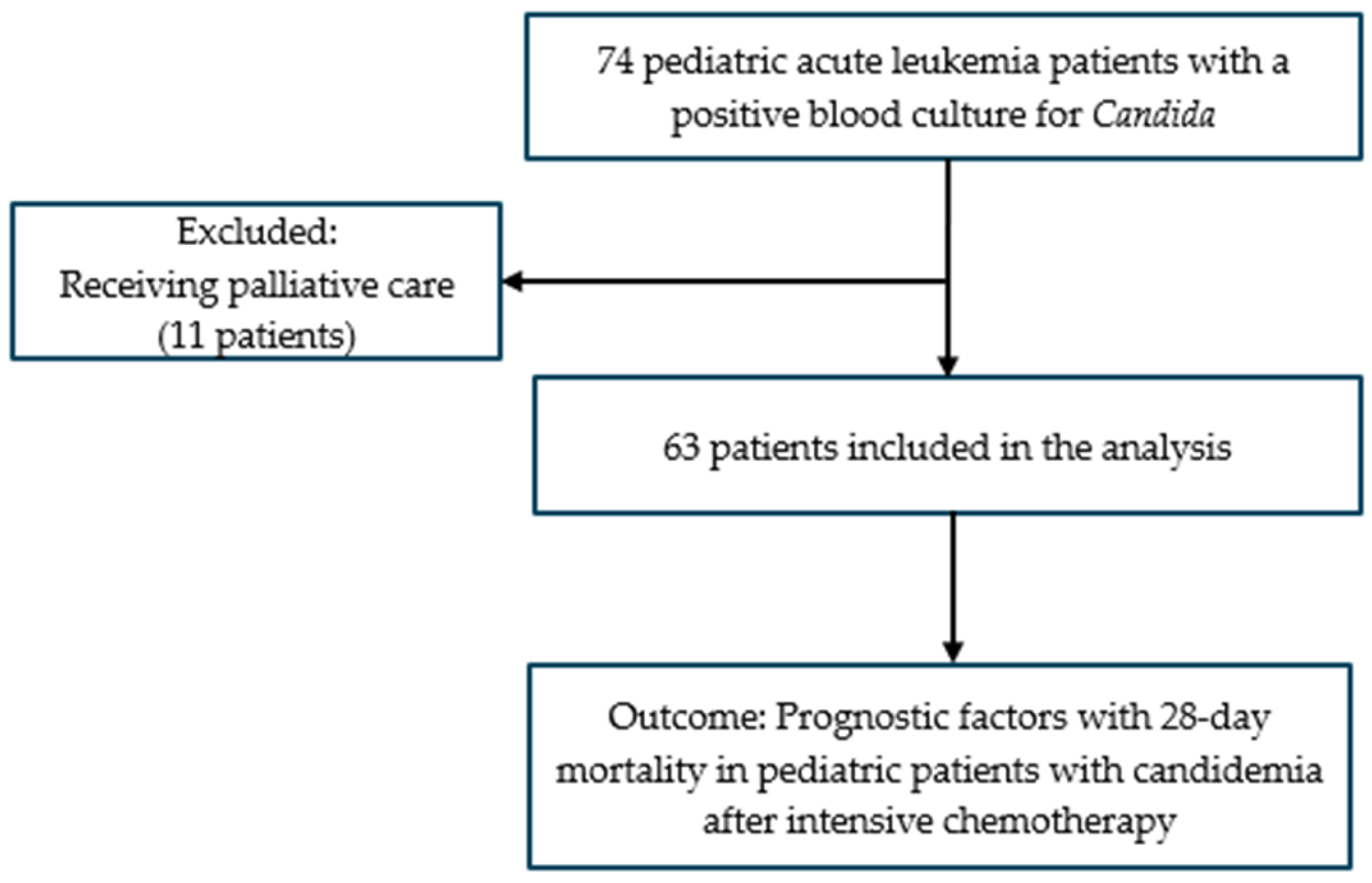
2. Materials and Methods
2.1. Study Design
2.2. Candidemia Diagnosis and Data Collection
2.3. Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFP | Antifungal prophylaxis |
| ALC | Absolute lymphocyte count |
| ALL | Acute lymphoblastic leukemia |
| AmB | Amphotericin B |
| AML | Acute myeloid leukemia |
| ANC | Absolute neutrophil count |
| BMI | Body mass index |
| CAS | Caspofungin |
| CI | Confidence interval |
| CVCs | Central venous catheters |
| G-CSF | Granulocyte colony-stimulating factor |
| GTX | Granulocyte transfusions |
| HR | Hazard ratio |
| IFI | Invasive fungal infection |
| R/R | Relapsed/refractory |
| Th17 | T-helper 17 |
| TRM | Treatment-related mortality |
References
- Reinhardt, D.; Antoniou, E.; Waack, K. Pediatric Acute Myeloid Leukemia—Past, Present, and Future. J. Clin. Med. 2022, 11, 504. [Google Scholar] [CrossRef]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, B.S.; McNeil, M.J.; Pham, L.T.D.; Chen, Y.; Rivera, J.; Acuna, C.; Sniderman, L.; Sakaan, F.M.; Aceituno, A.M.; Villegas, C.A.; et al. Treatment-Related Mortality in Children with Cancer in Low-Income and Middle-Income Countries: A Systematic Review and Meta-Analysis. Lancet Oncol. 2023, 24, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.B.; Wang, L.; Inaba, H.; Triplett, B.M.; Pounds, S.; Ribeiro, R.C.; Pui, C.; Rubnitz, J.E. Decreased Relapsed Rate and Treatment-related Mortality Contribute to Improved Outcomes for Pediatric Acute Myeloid Leukemia in Successive Clinical Trials. Cancer 2017, 123, 3791–3798. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, S.; Tridello, G.; Castagnola, E.; Calore, E.; Carraro, F.; Mariotti, I.; Colombini, A.; Perruccio, K.; Decembrino, N.; Russo, G.; et al. Retrospective Study on the Incidence and Outcome of Proven and Probable Invasive Fungal Infections in High-risk Pediatric Onco-hematological Patients. Eur. J. Haematol. 2017, 99, 240–248. [Google Scholar] [CrossRef]
- Groll, A.H.; Castagnola, E.; Cesaro, S.; Dalle, J.-H.; Engelhard, D.; Hope, W.; Roilides, E.; Styczynski, J.; Warris, A.; Lehrnbecher, T. Fourth European Conference on Infections in Leukaemia (ECIL-4): Guidelines for Diagnosis, Prevention, and Treatment of Invasive Fungal Diseases in Paediatric Patients with Cancer or Allogeneic Haemopoietic Stem-Cell Transplantation. Lancet Oncol. 2014, 15, e327–e340. [Google Scholar] [CrossRef]
- Groll, A.H.; Pana, D.; Lanternier, F.; Mesini, A.; Ammann, R.A.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Engelhard, D.; Garcia-Vidal, C.; et al. 8th European Conference on Infections in Leukaemia: 2020 Guidelines for the Diagnosis, Prevention, and Treatment of Invasive Fungal Diseases in Paediatric Patients with Cancer or Post-Haematopoietic Cell Transplantation. Lancet Oncol. 2021, 22, e254–e269. [Google Scholar] [CrossRef]
- Shoham, S.; Levitz, S.M. The Immune Response to Fungal Infections. Br. J. Haematol. 2005, 129, 569–582. [Google Scholar] [CrossRef]
- Drummond, R.A.; Desai, J.V.; Ricotta, E.E.; Swamydas, M.; Deming, C.; Conlan, S.; Quinones, M.; Matei-Rascu, V.; Sheriff, L.; Lecky, D.; et al. Long-Term Antibiotic Exposure Promotes Mortality after Systemic Fungal Infection by Driving Lymphocyte Dysfunction and Systemic Escape of Commensal Bacteria. Cell Host Microbe 2022, 30, 1020–1033.e6. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Netelenbos, T.; Massey, E.; De Wreede, L.C.; Harding, K.; Hamblin, A.; Sekhar, M.; Li, A.; Ypma, P.F.; Ball, L.; Zwaginga, J.J.; et al. The Burden of Invasive Infections in Neutropenic Patients: Incidence, Outcomes, and Use of Granulocyte Transfusions. Transfusion 2019, 59, 160–168. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Gea-Banacloche, J.; Stroncek, D.; Kadri, S.S. Granulocyte Transfusions in the Management of Invasive Fungal Infections. Br. J. Haematol. 2017, 177, 357–374. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J.; De Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. Management of Febrile Neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27, v111–v118. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.H.; Wingard, J.R. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group; De Onis, M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006, 95, 76–85. [Google Scholar] [CrossRef]
- De Onis, M. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Gökbuget, N.; Boissel, N.; Chiaretti, S.; Dombret, H.; Doubek, M.; Fielding, A.; Foà, R.; Giebel, S.; Hoelzer, D.; Hunault, M.; et al. Management of ALL in Adults: 2024 ELN Recommendations from a European Expert Panel. Blood 2024, 143, 1903–1930. [Google Scholar] [CrossRef]
- Pana, Z.D.; Roilides, E.; Warris, A.; Groll, A.H.; Zaoutis, T. Epidemiology of Invasive Fungal Disease in Children. J. Pediatr. Infect. Dis. Soc. 2017, 6, S3–S11. [Google Scholar] [CrossRef]
- Iyadorai, T.; Tay, S.T.; Liong, C.C.; Samudi, C.; Chow, L.C.; Cheong, C.S.; Velayuthan, R.; Tan, S.M.; Gan, G.G. A review of the epidemiology of invasive fungal infections in Asian patients with hematological malignancies (2011–2021). Epidemiol. Rev. 2024, 46, 1–12. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida Albicans and Emerging Non-Albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef]
- Tan, T.Y.; Hsu, L.Y.; Alejandria, M.M.; Chaiwarith, R.; Chinniah, T.; Chayakulkeeree, M.; Choudhury, S.; Chen, Y.H.; Shin, J.H.; Kiratisin, P.; et al. Antifungal Susceptibility of Invasive Candida Bloodstream Isolates from the Asia-Pacific Region. Med. Mycol. 2016, 54, 471–477. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Díaz-Martín, A.; García-Cabrera, E.; Ruiz Pérez De Pipaón, M.; Hernández-Caballero, C.; Aznar-Martín, J.; Cisneros, J.M.; Ortiz-Leyba, C. Risk Factors for Fluconazole-Resistant Candidemia. Antimicrob. Agents Chemother. 2010, 54, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Şanlı, K.; Arslantaş, E.; Ceylan, A.N.; Öncel, B.; Özkorucu, D.; Özkan Karagenç, A. Candidemia in Pediatric-Clinic: Frequency of Occurrence, Candida Species, Antifungal Susceptibilities, and Effects on Mortality (2020–2024). Diagnostics 2024, 14, 2343. [Google Scholar] [CrossRef]
- Kazakou, N.; Vyzantiadis, T.-A.; Gambeta, A.; Vasileiou, E.; Tsotridou, E.; Kotsos, D.; Giantsidi, A.; Saranti, A.; Palabougiouki, M.; Ioannidou, M.; et al. Invasive Fungal Infections in a Pediatric Hematology-Oncology Department: A 16-Year Retrospective Study. Curr. Med. Mycol. 2020, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Salmanton-García, J.; Egger, M.; Gangneux, J.-P.; Bicanic, T.; Arikan-Akdagli, S.; Alastruey-Izquierdo, A.; Klimko, N.; Barac, A.; Özenci, V.; et al. Guideline Adherence and Survival of Patients with Candidaemia in Europe: Results from the ECMM Candida III Multinational European Observational Cohort Study. Lancet Infect. Dis. 2023, 23, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Seidel, K.; Slavin, M.A.; Bowden, R.A.; Schoch, H.G.; Flowers, M.E.D.; Corey, L.; Boeckh, M. Prolonged Fluconazole Prophylaxis Is Associated with Persistent Protection against Candidiasis-Related Death in Allogeneic Marrow Transplant Recipients: Long-Term Follow-up of a Randomized, Placebo-Controlled Trial. Blood 2000, 96, 2055–2061. [Google Scholar] [CrossRef]
- Finfer, S.; Venkatesh, B.; Hotchkiss, R.S.; Sasson, S.C. Lymphopenia in Sepsis—An Acquired Immunodeficiency? Immunol. Cell Biol. 2023, 101, 535–544. [Google Scholar] [CrossRef]
- Drewry, A.M.; Samra, N.; Skrupky, L.P.; Fuller, B.M.; Compton, S.M.; Hotchkiss, R.S. Persistent Lymphopenia after Diagnosis of Sepsis Predicts Mortality. Shock 2014, 42, 383–391. [Google Scholar] [CrossRef]
- Ortega-Loubon, C.; Cano-Hernández, B.; Poves-Alvarez, R.; Muñoz-Moreno, M.F.; Román-García, P.; Balbás-Alvarez, S.; De La Varga-Martínez, O.; Gómez-Sánchez, E.; Gómez-Pesquera, E.; Lorenzo-López, M.; et al. The Overlooked Immune State in Candidemia: A Risk Factor for Mortality. J. Clin. Med. 2019, 8, 1512. [Google Scholar] [CrossRef]
- Løhmann, D.J.; Asdahl, P.H.; Abrahamsson, J.; Ha, S.Y.; Jónsson, Ó.G.; Kaspers, G.J.; Koskenvuo, M.; Lausen, B.; De Moerloose, B.; Palle, J.; et al. Use of granulocyte colony-stimulating factor and risk of relapse in pediatric patients treated for acute myeloid leukemia according to NOPHO-AML 2004 and DB AML-01. Pediatr. Blood Cancer 2019, 66, e27701. [Google Scholar] [CrossRef]
- Ehlers, S.; Herbst, C.; Zimmermann, M.; Scharn, N.; Germeshausen, M.; von Neuhoff, N.; Zwaan, C.M.; Reinhardt, K.; Hollink, I.H.; Klusmann, J.H.; et al. Granulocyte Colony-Stimulating Factor (G-CSF) Treatment of Childhood Acute Myeloid Leukemias That Overexpress the Differentiation-Defective G-CSF Receptor Isoform IV Is Associated with a Higher Incidence of Relapse. J. Clin. Oncol. 2010, 28, 2591–2597. [Google Scholar] [CrossRef]
- Arad-Cohen, N.; Zeller, B.; Abrahamsson, J.; Navarro, J.M.F.; Cheuk, D.; Palmu, S.; Costa, V.; De Moerloose, B.; Hasle, H.; Jahnukainen, K.; et al. Supportive care in pediatric acute myeloid leukemia:Expert-based recommendations of the NOPHO-DB-SHIP consortium. Expert Rev. Anticancer Ther. 2022, 22, 1183–1196. [Google Scholar] [CrossRef]
- Price, T.H.; Boeckh, M.; Harrison, R.W.; McCullough, J.; Ness, P.M.; Strauss, R.G.; Nichols, W.G.; Hamza, T.H.; Cushing, M.M.; King, K.E.; et al. Efficacy of Transfusion with Granulocytes from G-CSF/Dexamethasone–Treated Donors in Neutropenic Patients with Infection. Blood 2015, 126, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Cugno, C.; Deola, S.; Filippini, P.; Stroncek, D.F.; Rutella, S. Granulocyte Transfusions in Children and Adults with Hematological Malignancies: Benefits and Controversies. J. Transl. Med. 2015, 13, 362. [Google Scholar] [CrossRef]
| Characteristics | n | % | |
|---|---|---|---|
| Sex | - | - | |
| Male | 36 | 57.1 | |
| Female | 27 | 42.9 | |
| Age (years), median 9.7 (1.3–15.9) | - | - | |
| ≥10 | 30 | 47.6 | |
| <10 | 33 | 52.4 | |
| BMI | - | - | |
| Overweight | 7 | 11.1 | |
| Normal | 45 | 71.4 | |
| Underweight | 11 | 17.5 | |
| Leukemia status | - | - | |
| R/R disease | AML | 7 | 11.1 |
| ALL | 21 | 33.3 | |
| Induction phase | AML | 5 | 7.9 |
| ALL | 11 | 17.5 | |
| Complete remission | AML | 17 | 27 |
| ALL | 2 | 3.2 | |
| Chemotherapy regimen | - | - | |
| FLAG ± Dauno | 26 | 41.3 | |
| FRALLE 2000 B/T Induction | 10 | 15.9 | |
| 3 + 7; ADE | 10 | 15.9 | |
| MiDAC; HiDAC | 8 | 12.7 | |
| COOPRALL 2007-Vanda | 4 | 6.3 | |
| Others | 5 | 7.9 | |
| Characteristics | n | % | Median (Min–Max) |
|---|---|---|---|
| Candida spp. | - | - | - |
| C. tropicalis | 61 | 96.8 | - |
| C. albicans | 1 | 1.6 | - |
| C. krusei | 1 | 1.6 | - |
| Antifungal susceptibility (n = 60) | - | - | - |
| Fluconazole | 14 | 22.2 | - |
| Amphotericin B | 60 | 100 | - |
| Caspofungin | 60 | 100 | - |
| Antifungal prophylaxis | - | ||
| Fluconazole | 27 | 42.9 | - |
| Itraconazole | 11 | 17.5 | - |
| No prophylaxis | 25 | 39.7 | - |
| Primary candidemia treatment | - | ||
| CAS | 44 | 69.8 | - |
| AmB | 15 | 23.8 | - |
| VOR + CAS | 2 | 3.2 | - |
| Fluconazole (Intravenous) | 2 | 3.2 | - |
| Granulocyte transfusions | 6 units (2–12) | ||
| GTX + G-CSF | 25 | 39.7 | - |
| G-CSF alone | 38 | 60.3 | - |
| ANC at candidemia diagnosis | 0.01 G/L (0–0.85) | ||
| <0.1 G/L | 61 | 96.8 | - |
| 0.5–1 G/L | 2 | 3.2 | - |
| ALC at candidemia diagnosis | 0.12 G/L (0–1.03) | ||
| ≥0.2 G/L | 19 | 30.2 | - |
| <0.2 G/L | 44 | 69.8 | - |
| Concomitant bacterial infection | - | ||
| Yes | 6 | 9.5 | - |
| No | 57 | 90.5 | - |
| Factors | 28-Day Mortality n (%) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR [95% CI] | p | HR [95% CI] | p | ||
| Fluconazole susceptibility (n = 60) | |||||
| Sensitive (n = 14) | 2 (14.3) | 0.3 [0.07–1.31] | 0.11 | ||
| Resistant (n = 46) | 21 (42.9) | ||||
| Antifungal prophylaxis | |||||
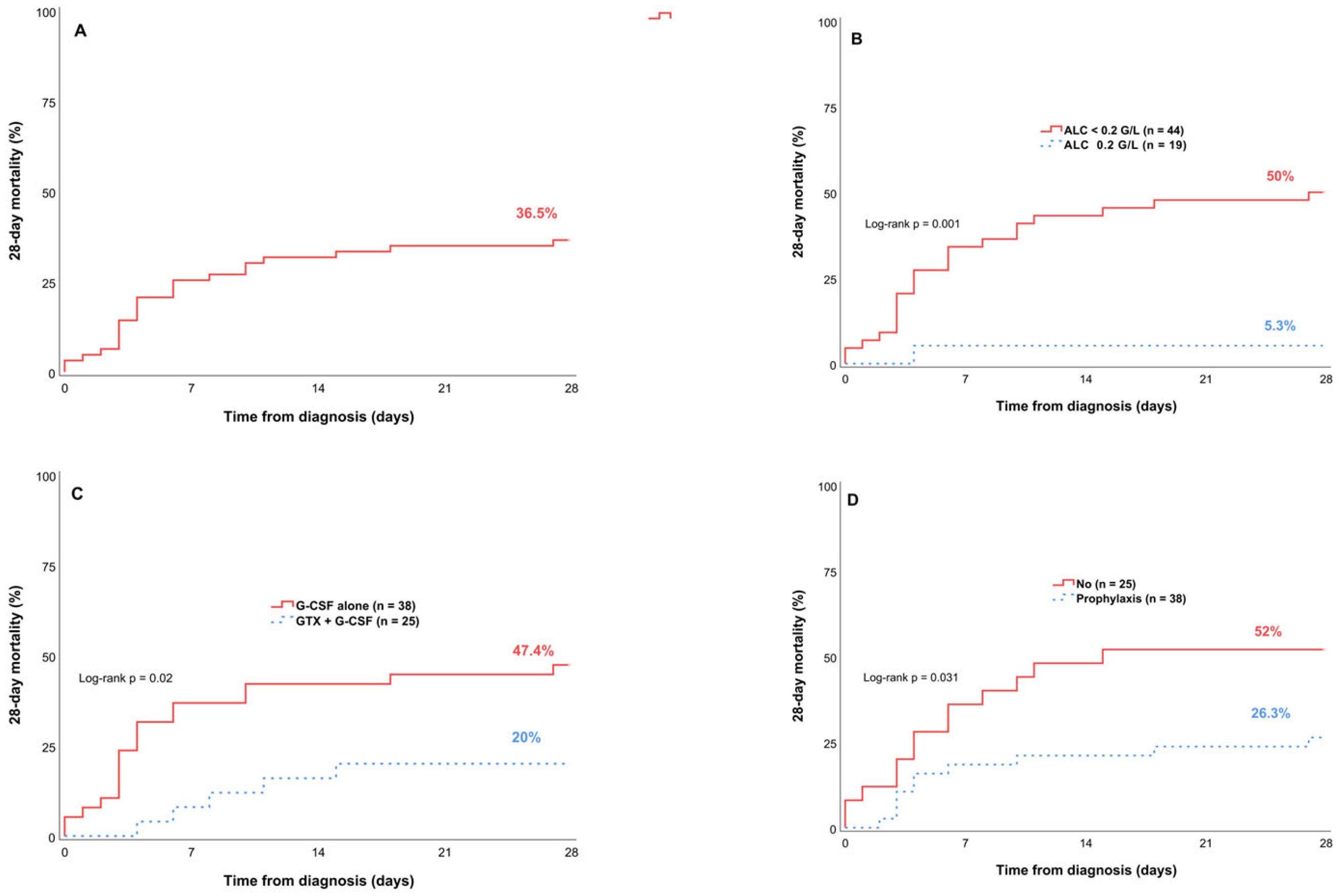
| Yes (n = 38) | 10 (26.3) | 0.42 [0.18–0.96] | 0.04 | 0.31 [0.13–0.74] | 0.008 |
| No (n = 25) | 13 (52) | ||||
| Prophylactic antifungal agents (n = 38) | |||||
| Fluconazole (n = 27) | 8 (29.6) | 1.85 [0.39–8.76] | 0.433 | ||
| Itraconazole (n = 11) | 2 (18.2) | ||||
| Primary treatment (n = 59) | |||||
| AmB (n = 15) | 4 (26.7) | 0.59 [0.2–1.76] | 0.353 | ||
| CAS (n = 44) | 18 (40.9) | ||||
| Granulocyte transfusions | |||||
| GTX + G-CSF (n = 25) | 5 (20) | 0.33 [0.12–0.9] | 0.03 | 0.31 [0.11–0.85] | 0.024 |
| G-CSF alone (n = 38) | 18 (47.4) | ||||
| ALC at candidemia diagnosis | |||||
| ALC ≥ 0.2 G/L (n = 19) | 1 (5.3) | 0.08 [0.01–0.6] | 0.014 | 0.08 [0.01–0.61] | 0.015 |
| ALC < 0.2 G/L (n = 44) | 22 (50) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
My, T.T.K.; Hong, H.T.; Lan, M.; Mai, T.Q.; Hai, D.H.; Ngan, T.T.D. Prognostic Factors for 28-Day Mortality in Pediatric Patients with Acute Leukemia and Candidemia Following Intensive Chemotherapy: A Retrospective Study. Hematol. Rep. 2025, 17, 38. https://doi.org/10.3390/hematolrep17040038
My TTK, Hong HT, Lan M, Mai TQ, Hai DH, Ngan TTD. Prognostic Factors for 28-Day Mortality in Pediatric Patients with Acute Leukemia and Candidemia Following Intensive Chemotherapy: A Retrospective Study. Hematology Reports. 2025; 17(4):38. https://doi.org/10.3390/hematolrep17040038
Chicago/Turabian StyleMy, Tran Thi Kieu, Hoang Thi Hong, Mai Lan, Tran Quynh Mai, Dang Hoang Hai, and Ta Thi Dieu Ngan. 2025. "Prognostic Factors for 28-Day Mortality in Pediatric Patients with Acute Leukemia and Candidemia Following Intensive Chemotherapy: A Retrospective Study" Hematology Reports 17, no. 4: 38. https://doi.org/10.3390/hematolrep17040038
APA StyleMy, T. T. K., Hong, H. T., Lan, M., Mai, T. Q., Hai, D. H., & Ngan, T. T. D. (2025). Prognostic Factors for 28-Day Mortality in Pediatric Patients with Acute Leukemia and Candidemia Following Intensive Chemotherapy: A Retrospective Study. Hematology Reports, 17(4), 38. https://doi.org/10.3390/hematolrep17040038






