Management of Refractory Malignancy-Associated Hemophagocytic Lymphohistiocytosis in Adolescent Patients: A Case Series of Novel Therapeutics and Treatment Challenges
Abstract
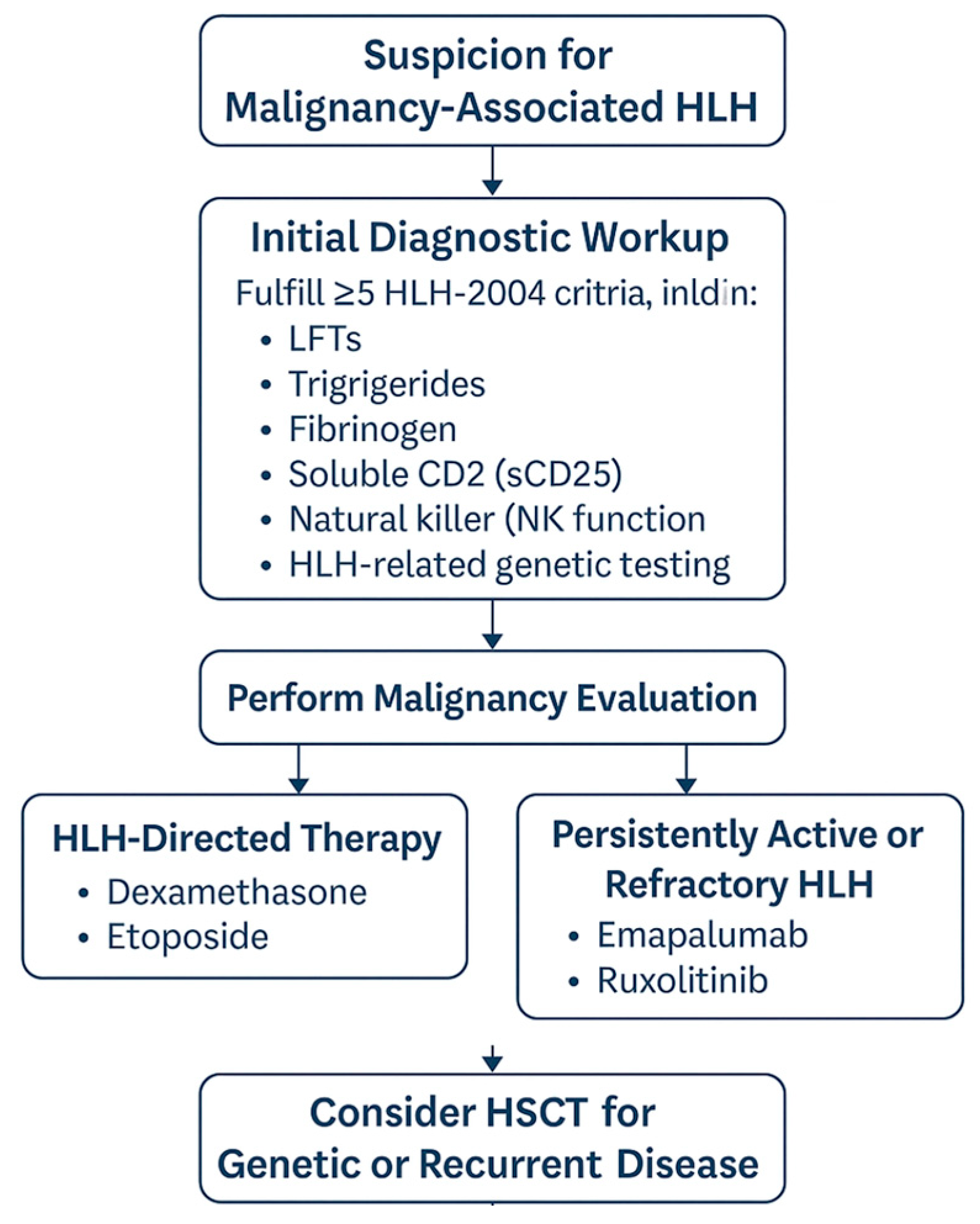
1. Introduction
- The initial diagnostic approach, including whether and when to perform bone marrow biopsy, flow cytometry, or genetic testing for HLH-related mutations.
- The treatment focus: whether to prioritize HLH-directed immunosuppression or start malignancy-targeted chemotherapy first.
- The modification of standard HLH treatment protocols in cases where chemotherapeutic toxicities may further compromise an already critically ill patient.
2. Patient Cases
2.1. Patient Cases
2.1.1. Patient 1
2.1.2. Patient 2
2.1.3. Patient 3
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henter, J.I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lehmberg, K.; E Nichols, K.; Henter, J.-I.; Girschikofsky, M.; Greenwood, T.; Jordan, M.; Kumar, A.; Minkov, M.; La Rosée, P.; Weitzman, S.; et al. Consensus recommendations for the diagnosis and management of hemophagocytic lymphohistiocytosis associated with malignancies. Haematologica 2015, 100, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, C.; Marafon, D.P.; Caiello, I.; de Graaf, K.; Guilhot, F.; Ferlin, W.; Davì, S.; Schulert, G.; Ravelli, A.; Grom, A.; et al. High levels of interferon-gamma (IFNγ) in macrophage activation syndrome (MAS) and CXCL9 levels as a biomarker for IFNγ production in MAS. Pediatr. Rheumatol. Online J. 2015, 13 (Suppl. 1), O84. [Google Scholar] [CrossRef][Green Version]
- Tabata, C.; Tabata, R. Possible prediction of underlying lymphoma by high sIL-2R/ferritin ratio in hemophagocytic syndrome. Ann. Hematol. 2012, 91, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Jordan, M.B.; Allen, C.; Cesaro, S.; Rizzari, C.; Rao, A.; Degar, B.; Garrington, T.P.; Sevilla, J.; Putti, M.C.; et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2020, 382, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Teachey, D.T.; Devidas, M.; Wood, B.L.; Chen, Z.; Hayashi, R.J.; Hermiston, M.L.; Annett, R.D.; Archer, J.H.; Asselin, B.L.; August, K.J.; et al. Children’s Oncology Group Trial AALL1231: A phase III clinical trial testing bortezomib in newly diagnosed T-cell acute lymphoblastic leukemia and lymphoma. J. Clin. Onc. 2022, 40, 2106–2118. [Google Scholar] [CrossRef] [PubMed]
- Teachey, D.T.; Hunger, S.P. Predicting relapse risk in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2013, 162, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Wu, L.; Wang, X.; Jin, Z.; Gao, Z.; Wang, Z. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica 2020, 105, e210–e212. [Google Scholar] [CrossRef] [PubMed]
- van Enckevort, F.H.; Netea, M.G.; Hermus, A.R.; Sweep, C.G.; Meis, J.F.; Van der Meer, J.W.; Kullberg, B.J. Increased susceptibility to systemic candidiasis in interleukin-6 deficient mice. Med. Mycol. 1999, 37, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Knauft, J.; Schenk, T.; Ernst, T.; Schnetzke, U.; Hochhaus, A.; La Rosée, P.; Birndt, S. Lymphoma-associated hemophagocytic lymphohistiocytosis (LA-HLH): A scoping review unveils clinical and diagnostic patterns of a lymphoma subgroup with poor prognosis. Leukemia 2024, 38, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and validation of the HScore to diagnose reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Kim, J.Y.; Kim, M.H.; Park, J.K.; Lee, E.Y.; Lee, E.B.; Park, J.W. Successful treatment of hemophagocytic lymphohistiocytosis in a patient with systemic lupus erythematosus with ruxolitinib: A case report. J. Rheum. Dis. 2023, 31, 125–129. [Google Scholar] [CrossRef] [PubMed]
- La Rosée, P.; Horne, A.; Hines, M.; von Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
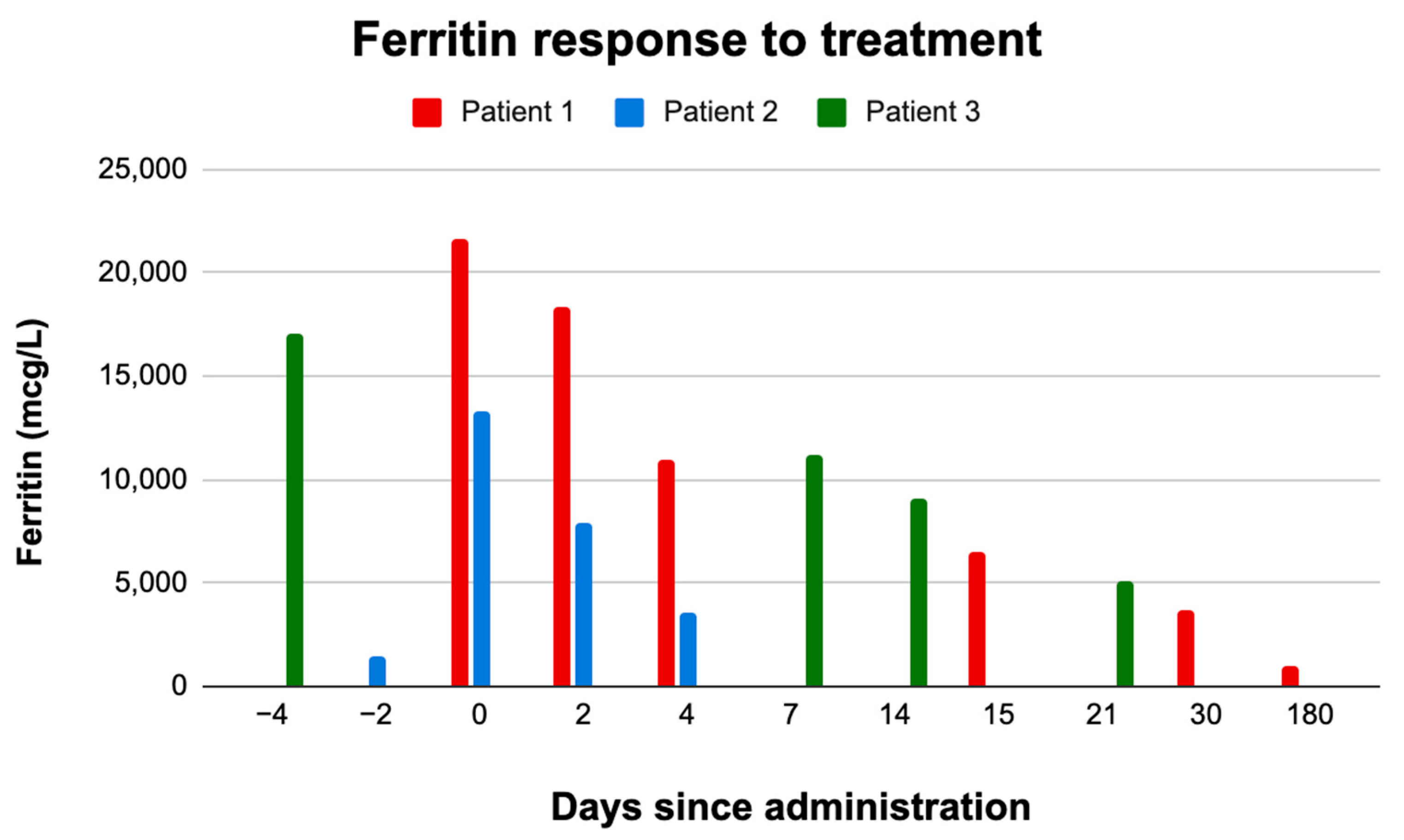
| Patient 1 | Patient 2 ** | Patient 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days since administration | 0 | 2 | 4 | 15 | 30 | 180 | −2 | 0 | 2 | 4 | −4 | 0 | 7 | 14 | 21 |
| Tmax | 36.1 | 36.2 | 36.5 | 36.4 | 36.4 | 36.4 | 39.3 | 37.4 | 36.7 | 38.4 | 36.7 | 36.4 | 36.4 | 36.4 | 36.3 |
| wbc | 33.2 | 25 | 12.4 | 1.5 | 21.2 | 4.9 | 0.1 | 0.1 | 0.1 | 0.1 | 1.5 | 0.9 | 1.6 | 2.9 | 1.5 |
| Hgb | 7.5 | 7.1 | 8.9 | 8.6 | 10.6 | 13.2 | 7.3 | 7.9 | 8.3 | 5.4 | 7.3 | 8.1 | 7.5 | 7.6 | 9.1 |
| Hct | 23.4 | 21.1 | 26.4 | 25.3 | 32 | 38.8 | 20.5 | 23.2 | 23.3 | 15.4 | 21.7 | 24 | 22.6 | 22.5 | 25.9 |
| Mcv | 84.3 | 84.1 | 86.3 | 86.3 | 94.9 | 101.7 | 80.1 | 81.7 | 79.8 | 78.4 | 97.1 | 93.5 | 96.2 | 98.9 | 99.7 |
| Plt | 833 | 524 | 391 | 242 | 449 | 215 | 13 | 19 | 44 | 25 | 25 | 25 | 21 | 33 | 29 |
| Ferritin | 21,627 | 18,359 | 10,949 | 6470 | 3714 | 1046 | 1459 | 13,279 | 7889 | 3604 | 17,094 | * | 11,139 | 9081 | 5153 |
| AST | 63 | 50 | 84 | 60 | * | 40 | 618 | 421 | 251 | 68 | 220 | 204 | 155 | 133 | 77 |
| ALT | 89 | 80 | 178 | 190 | * | 45 | 240 | 185 | 137 | 37 | 427 | 364 | 259 | 213 | 168 |
| TG | * | * | * | * | * | * | 595 | 1188 | 1890 | 814 | 476 | * | * | * | * |
| Fibrinogen | * | * | * | * | * | * | 101 | 135 | 155 | 194 | 430 | * | 349 | 430 | 438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnareddigari, M.; Vo, K.; Panigrahi, A. Management of Refractory Malignancy-Associated Hemophagocytic Lymphohistiocytosis in Adolescent Patients: A Case Series of Novel Therapeutics and Treatment Challenges. Hematol. Rep. 2025, 17, 28. https://doi.org/10.3390/hematolrep17030028
Krishnareddigari M, Vo K, Panigrahi A. Management of Refractory Malignancy-Associated Hemophagocytic Lymphohistiocytosis in Adolescent Patients: A Case Series of Novel Therapeutics and Treatment Challenges. Hematology Reports. 2025; 17(3):28. https://doi.org/10.3390/hematolrep17030028
Chicago/Turabian StyleKrishnareddigari, Meha, Kenny Vo, and Arun Panigrahi. 2025. "Management of Refractory Malignancy-Associated Hemophagocytic Lymphohistiocytosis in Adolescent Patients: A Case Series of Novel Therapeutics and Treatment Challenges" Hematology Reports 17, no. 3: 28. https://doi.org/10.3390/hematolrep17030028
APA StyleKrishnareddigari, M., Vo, K., & Panigrahi, A. (2025). Management of Refractory Malignancy-Associated Hemophagocytic Lymphohistiocytosis in Adolescent Patients: A Case Series of Novel Therapeutics and Treatment Challenges. Hematology Reports, 17(3), 28. https://doi.org/10.3390/hematolrep17030028





