The Real-World Outcomes of Relapsed/Refractory Multiple Myeloma Treated with Elotuzumab, Pomalidomide, and Dexamethasone
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Treatment and Dose Adjustment
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Treatment Exposure
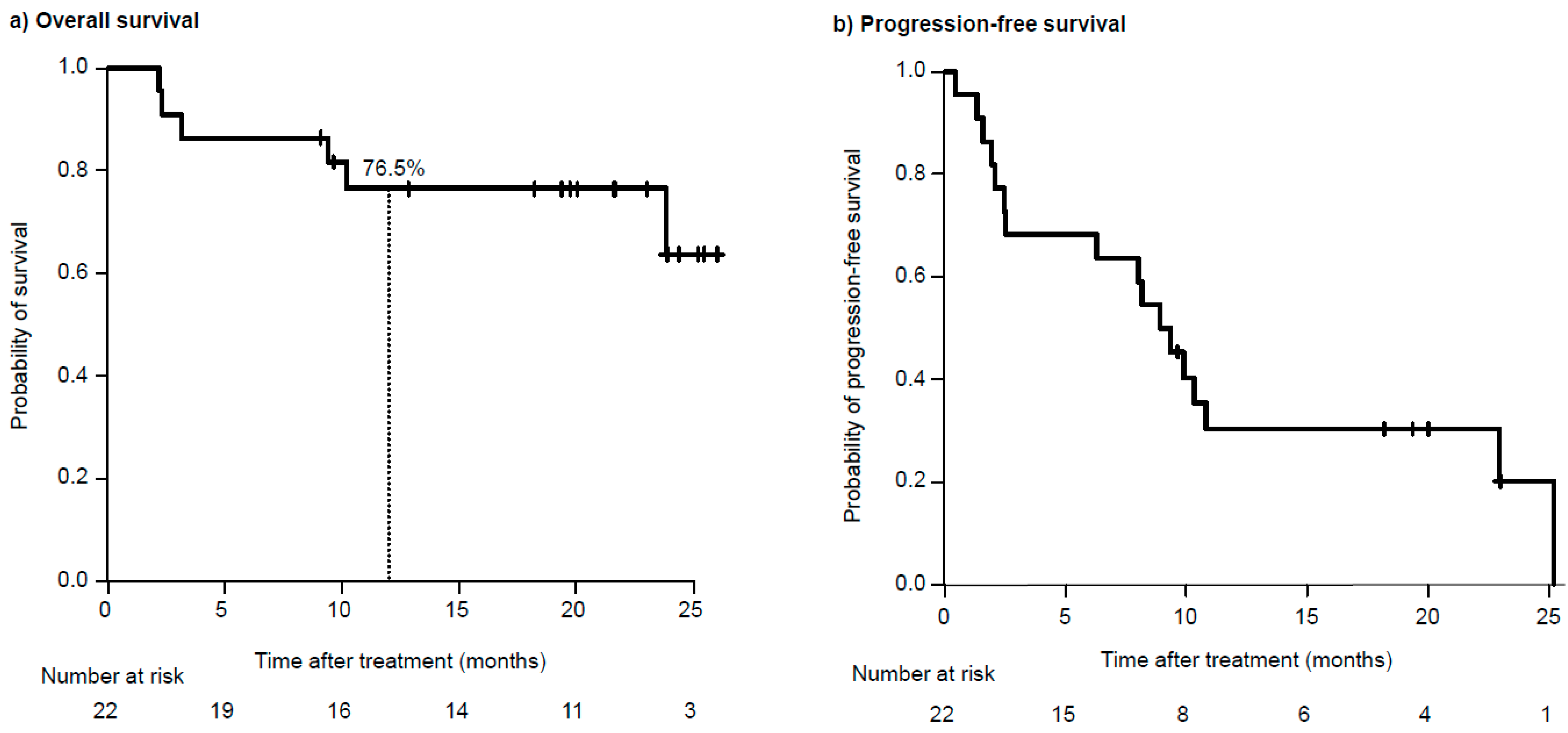
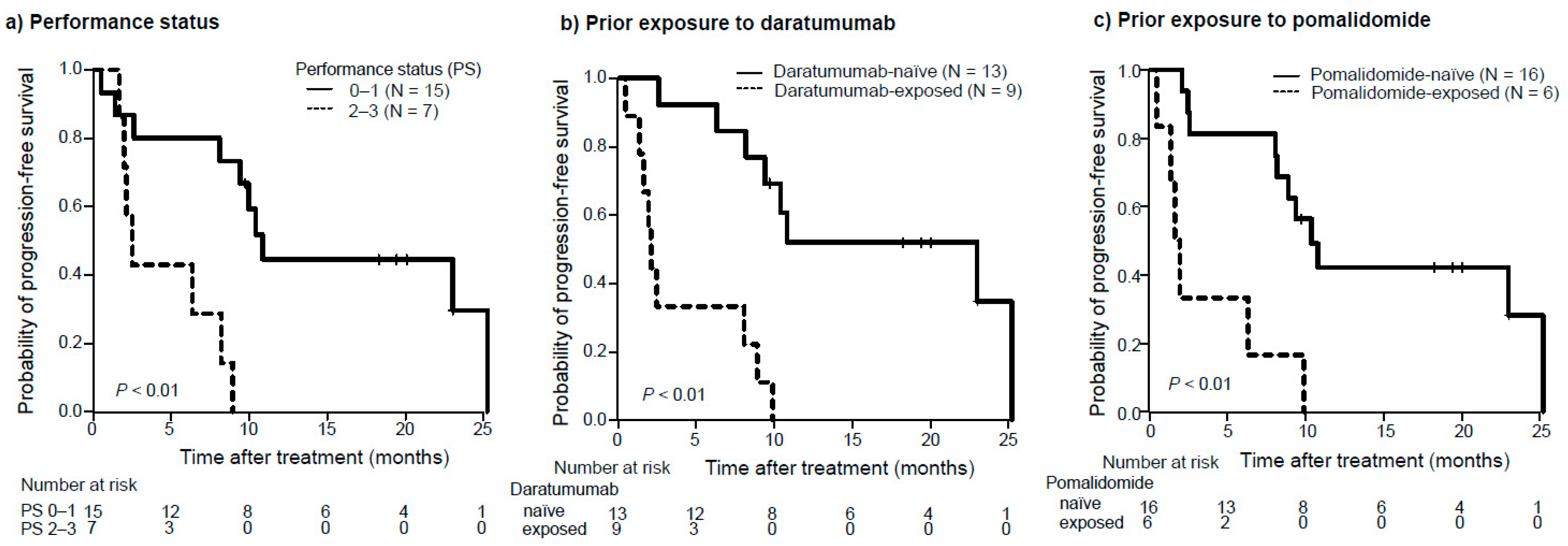
3.2. Efficacy
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyle, R.A.; Rajkumar, S.V. Multiple myeloma. N. Engl. J. Med. 2004, 351, 1860–1873. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef]
- Sonneveld, P.; Goldschmidt, H.; Rosinol, L.; Blade, J.; Lahuerta, J.J.; Cavo, M.; Tacchetti, P.; Zamagni, E.; Attal, M.; Lokhorst, H.M.; et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: A meta-analysis of phase III randomized, controlled trials. J. Clin. Oncol. 2013, 31, 3279–3287. [Google Scholar] [CrossRef]
- Gay, F.; Larocca, A.; Wijermans, P.; Cavallo, F.; Rossi, D.; Schaafsma, R.; Genuardi, M.; Romano, A.; Liberati, A.M.; Siniscalchi, A.; et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: Analysis of 1175 patients. Blood 2011, 117, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Anderson, W.F.; Landgren, O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia 2014, 28, 1346–1348. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Dickman, P.W.; Landgren, O.; Blimark, C.; Hultcrantz, M.; Turesson, I.; Bjorkholm, M.; Kristinsson, S.Y. Dramatically improved survival in multiple myeloma patients in the recent decade: Results from a Swedish population-based study. Haematologica 2018, 103, e412–e415. [Google Scholar] [CrossRef]
- Kumar, S.; Williamson, M.; Ogbu, U.; Surinach, A.; Arndorfer, S.; Hong, W.J. Front-line treatment patterns in multiple myeloma: An analysis of U.S.-based electronic health records from 2011 to 2019. Cancer Med. 2021, 10, 5866–5877. [Google Scholar] [CrossRef]
- Usmani, S.; Ahmadi, T.; Ng, Y.; Lam, A.; Desai, A.; Potluri, R.; Mehra, M. Analysis of Real-World Data on Overall Survival in Multiple Myeloma Patients with >/=3 Prior Lines of Therapy Including a Proteasome Inhibitor (PI) and an Immunomodulatory Drug (IMiD), or Double Refractory to a PI and an IMiD. Oncologist 2016, 21, 1355–1361. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dimopoulos, M.A.; Kastritis, E.; Terpos, E.; Nahi, H.; Goldschmidt, H.; Hillengass, J.; Leleu, X.; Beksac, M.; Alsina, M.; et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 2017, 31, 2443–2448. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Hsi, E.D.; Steinle, R.; Balasa, B.; Szmania, S.; Draksharapu, A.; Shum, B.P.; Huseni, M.; Powers, D.; Nanisetti, A.; Zhang, Y.; et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin. Cancer Res. 2008, 14, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Dillon, M.; Song, W.; Leiba, M.; Li, X.F.; Burger, P.; Lee, A.I.; Podar, K.; Hideshima, T.; Rice, A.G.; et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008, 112, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Balasa, B.; Yun, R.; Belmar, N.A.; Fox, M.; Chao, D.T.; Robbins, M.D.; Starling, G.C.; Rice, A.G. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-alpha pathways. Cancer Immunol. Immunother. 2015, 64, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef]
- Pazina, T.; James, A.M.; MacFarlane, A.W.t.; Bezman, N.A.; Henning, K.A.; Bee, C.; Graziano, R.F.; Robbins, M.D.; Cohen, A.D.; Campbell, K.S. The anti-SLAMF7 antibody elotuzumab mediates NK cell activation through both CD16-dependent and -independent mechanisms. Oncoimmunology 2017, 6, e1339853. [Google Scholar] [CrossRef]
- Kurdi, A.T.; Glavey, S.V.; Bezman, N.A.; Jhatakia, A.; Guerriero, J.L.; Manier, S.; Moschetta, M.; Mishima, Y.; Roccaro, A.; Detappe, A.; et al. Antibody-Dependent Cellular Phagocytosis by Macrophages is a Novel Mechanism of Action of Elotuzumab. Mol. Cancer Ther. 2018, 17, 1454–1463. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab Plus Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma: Final Overall Survival Analysis from the Randomized Phase II ELOQUENT-3 Trial. J. Clin. Oncol. 2023, 41, 568–578. [Google Scholar] [CrossRef]
- Shah, J.J.; Abonour, R.; Gasparetto, C.; Hardin, J.W.; Toomey, K.; Narang, M.; Srinivasan, S.; Kitali, A.; Zafar, F.; Flick, E.D.; et al. Analysis of Common Eligibility Criteria of Randomized Controlled Trials in Newly Diagnosed Multiple Myeloma Patients and Extrapolating Outcomes. Clin. Lymphoma Myeloma Leuk. 2017, 17, 575–583.e572. [Google Scholar] [CrossRef]
- Knauf, W.; Aldaoud, A.; Hutzschenreuter, U.; Klausmann, M.; Dille, S.; Wetzel, N.; Janicke, M.; Marschner, N.; the TLN-Group (Tumour Registry Lymphatic Neoplasms). Survival of non-transplant patients with multiple myeloma in routine care differs from that in clinical trials-data from the prospective German Tumour Registry Lymphatic Neoplasms. Ann. Hematol. 2018, 97, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Romanus, D.; Palumbo, A.; Blazer, M.; Farrelly, E.; Raju, A.; Huang, H.; Richardson, P. Randomized Clinical Trial Representativeness and Outcomes in Real-World Patients: Comparison of 6 Hallmark Randomized Clinical Trials of Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 8–17.e16. [Google Scholar] [CrossRef] [PubMed]
- Larocca, A.; Dold, S.M.; Zweegman, S.; Terpos, E.; Wasch, R.; D’Agostino, M.; Scheubeck, S.; Goldschmidt, H.; Gay, F.; Cavo, M.; et al. Patient-centered practice in elderly myeloma patients: An overview and consensus from the European Myeloma Network (EMN). Leukemia 2018, 32, 1697–1712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Blade, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Hose, D.; Schreder, M.; Hefner, J.; Bittrich, M.; Danhof, S.; Strifler, S.; Krauth, M.T.; Schoder, R.; Gisslinger, B.; Einsele, H.; et al. Elotuzumab, pomalidomide, and dexamethasone is a very well tolerated regimen associated with durable remission even in very advanced myeloma: A retrospective study from two academic centers. J. Cancer Res. Clin. Oncol. 2021, 147, 205–212. [Google Scholar] [CrossRef]
- Gentile, M.; Vigna, E.; Palmieri, S.; Galli, M.; Derudas, D.; Mina, R.; Della Pepa, R.; Zambello, R.; Martino, E.A.; Bruzzese, A.; et al. Elotuzumab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma: A multicenter, retrospective, real-world experience with 200 cases outside of controlled clinical trials. Haematologica 2024, 109, 245–255. [Google Scholar] [CrossRef]
- Casneuf, T.; Xu, X.S.; Adams, H.C., 3rd; Axel, A.E.; Chiu, C.; Khan, I.; Ahmadi, T.; Yan, X.; Lonial, S.; Plesner, T.; et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017, 1, 2105–2114. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hughes, T.; Zhang, J.; Caligiuri, M.A.; Benson, D.M.; Yu, J. Fratricide of NK Cells in Daratumumab Therapy for Multiple Myeloma Overcome by Ex Vivo-Expanded Autologous NK Cells. Clin. Cancer Res. 2018, 24, 4006–4017. [Google Scholar] [CrossRef]
- Martino, E.A.; Palmieri, S.; Galli, M.; Derudas, D.; Mina, R.; Della Pepa, R.; Zambello, R.; Vigna, E.; Bruzzese, A.; Mangiacavalli, S.; et al. Elotuzumab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of a multicenter, retro-spective real-world experience with 321 cases outside of controlled clinical trials. Hematol. Oncol. 2024, 42, e3290. [Google Scholar] [CrossRef]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Kumar, S.K.; San Miguel, J.; Davies, F.; Zamagni, E.; Bahlis, N.; Ludwig, H.; Mikhael, J.; Terpos, E.; Schjesvold, F.; et al. Treatment of relapsed and refractory multiple myeloma: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2021, 22, e105–e118. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.; Giri, S.; Reagan, J.L.; Olszewski, A.J. Outcomes of lenalidomide- or bortezomib-based regimens in older patients with plasma cell myeloma. Am. J. Hematol. 2021, 96, 14–22. [Google Scholar] [CrossRef]
- Repetto, L.; Fratino, L.; Audisio, R.A.; Venturino, A.; Gianni, W.; Vercelli, M.; Parodi, S.; Dal Lago, D.; Gioia, F.; Monfardini, S.; et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J. Clin. Oncol. 2002, 20, 494–502. [Google Scholar] [CrossRef]
| Median Age, Years (Range) | 73.5 | (50–84) |
|---|---|---|
| Male sex, no (%) | 6 | (27) |
| ECOG performance status, no (%) | ||
| 0 | 12 | (55) |
| 1 | 3 | (14) |
| 2 | 4 | (18) |
| 3 | 3 | (14) |
| Type of myeloma, no (%) | ||
| IgG | 10 | (46) |
| IgA | 6 | (27) |
| Light chain | 6 | (27) |
| International Staging System (ISS) stage, no (%) | ||
| I or II | 11 | (50) |
| III | 11 | (50) |
| Cytogenetic abnormalities (Del17p, t(4;14), or t(14;16)), no (%) | ||
| Yes | 3 | (14) |
| No | 7 | (32) |
| Data not available | 12 | (55) |
| Extramedullary disease, no (%) | ||
| Yes | 3 | (14) |
| No | 19 | (86) |
| Amyloidosis, no (%) | ||
| Yes | 3 | (14) |
| No | 19 | (86) |
| Central nervous system involvement, no (%) | ||
| Yes | 0 | (0) |
| No | 22 | (22) |
| Comorbidities, no (%) | ||
| Heart failure | 3 | (14) |
| Chronic obstructive pulmonary disease | 2 | (9) |
| Other malignancy | 2 | (9) |
| Median no. of previous lines of therapy, (range) | 4 | (1–10) |
| Previous therapies, no (%) | ||
| Bortezomib | 21 | (96) |
| Lenalidomide | 18 | (82) |
| Daratumumab | 9 | (41) |
| Ixazomib | 9 | (41) |
| Pomalidomide | 6 | (27) |
| Carfilzomib | 3 | (14) |
| Isatuximab | 1 | (5) |
| Autologous stem cell transplantation | 10 | (46) |
| Allogeneic stem cell transplantation | 3 | (14) |
| Radiotherapy | 4 | (18) |
| Median time since initial diagnosis, months (range) | 52.6 | (4.7–129.6) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Factors | Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value |
| ECOG performance status | 6.3 | 1.8–22.4 | <0.01 | 4.1 | 1.1–15.6 | 0.04 |
| 0–1 versus 2–3 | ||||||
| Prior treatment with daratumumab | ||||||
| No versus Yes | 8.3 | 2.5–28.2 | <0.01 | 3.8 | 1.1–13.8 | 0.04 |
| Prior treatment with pomalidomide | ||||||
| No versus Yes | 5.8 | 1.9–17.9 | <0.01 | |||
| Events | Any Grade | Grade 3 or 4 | ||
|---|---|---|---|---|
| No. of Patients (%) | ||||
| Hematological adverse events | ||||
| Anemia | 20 | (91) | 5 | (23) |
| Neutropenia | 17 | (77) | 13 | (59) |
| Lymphocytopenia | 20 | (91) | 14 | (64) |
| Thrombocytopenia | 12 | (55) | 4 | (18) |
| Nonhematological adverse events | ||||
| Infection | 10 | (45) | 7 | (32) |
| Infusion reaction | 5 | (23) | 0 | (0) |
| Peripheral neuropathy | 5 | (23) | 0 | (0) |
| Skin rash | 3 | (14) | 0 | (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, H.; Aisa, Y.; Ito, C.; Sakurai, A.; Nakazato, T. The Real-World Outcomes of Relapsed/Refractory Multiple Myeloma Treated with Elotuzumab, Pomalidomide, and Dexamethasone. Hematol. Rep. 2024, 16, 593-602. https://doi.org/10.3390/hematolrep16040058
Nakayama H, Aisa Y, Ito C, Sakurai A, Nakazato T. The Real-World Outcomes of Relapsed/Refractory Multiple Myeloma Treated with Elotuzumab, Pomalidomide, and Dexamethasone. Hematology Reports. 2024; 16(4):593-602. https://doi.org/10.3390/hematolrep16040058
Chicago/Turabian StyleNakayama, Hitomi, Yoshinobu Aisa, Chisako Ito, Aki Sakurai, and Tomonori Nakazato. 2024. "The Real-World Outcomes of Relapsed/Refractory Multiple Myeloma Treated with Elotuzumab, Pomalidomide, and Dexamethasone" Hematology Reports 16, no. 4: 593-602. https://doi.org/10.3390/hematolrep16040058
APA StyleNakayama, H., Aisa, Y., Ito, C., Sakurai, A., & Nakazato, T. (2024). The Real-World Outcomes of Relapsed/Refractory Multiple Myeloma Treated with Elotuzumab, Pomalidomide, and Dexamethasone. Hematology Reports, 16(4), 593-602. https://doi.org/10.3390/hematolrep16040058







