Complete Remission with Inotuzumab Ozogamicin as Fourth-Line Salvage Therapy in a Child with Relapsed/Refractory Acute Lymphoblastic Leukemia
Abstract
1. Introduction
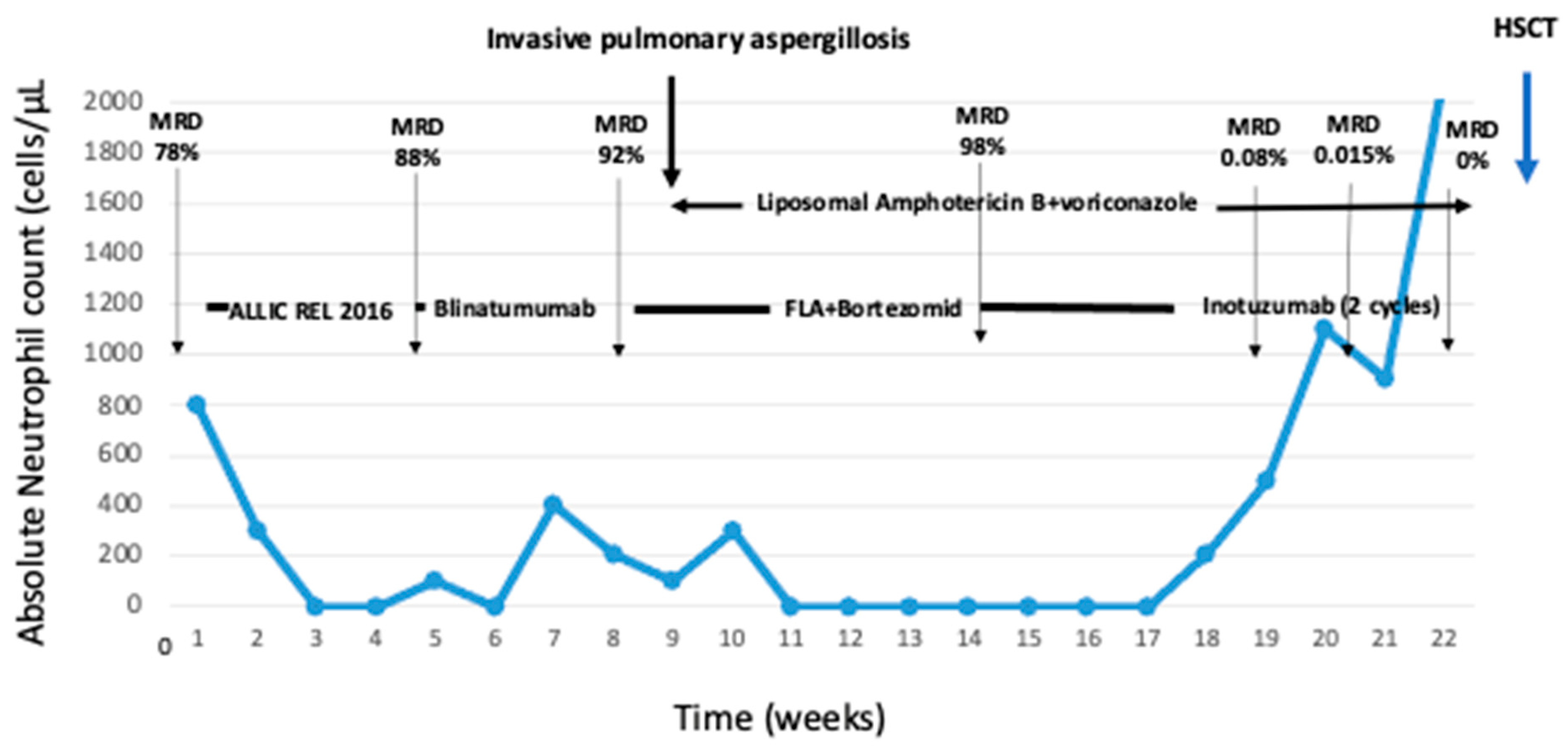
2. Detailed Case Description
3. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef]
- Pui, C.-H.; Mullighan, C.G.; Evans, W.E.; Relling, M.V. Pediatric acute lymphoblastic leukemia: Where are we going and how do we get there? Blood 2012, 120, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, T.; Soderhall, S.; Arvidson, J.; Forestier, E.; Montgomery, S.; Bottai, M.; Lausen, B.; Carlsen, N.; Hellebostad, M.; Lähteenmäki, P.; et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: Prognostic factors, treatment and outcome. Haematologica 2015, 101, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Rheingold, S.R.; Ji, L.; Xu, X.; Devidas, M.; Brown, P.A.; Gore, L.; Winick, N.J.; Carroll, W.L.; Hunger, S.; Raetz, E.A.; et al. Prognostic factors for survival after relapsed acute lymphoblastic leukemia (ALL): A Children’s Oncology Group (COG) study. J. Clin. Oncol. 2019, 37, 10008. [Google Scholar] [CrossRef]
- Dijoseph, J.; Armellino, D.; Boghaert, E.; Khandke, K.; Dougher, M.; Sridharan, L.; Kunz, A.; Hamann, P.R.; Gorovits, B.; Udata, C.; et al. Antibody-targeted chemotherapy with CMC-544: A CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 2004, 103, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Besponsa. Summary of Product Characteristics. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761040s003lbl.pdf (accessed on 22 July 2024).
- Balwierz, A.I. A Randomized Trial of the I-BFM-SG for the Management of Childhood Non-B Acute Lymphoblastic Leukemia. Final Version of Therapy Protocol from 14 August 2009. Available online: http://www.bialaczka.org/wp-content/uploads/2016/10/ALLIC_BFM_2009.pdf (accessed on 10 September 2024).
- ALL-IC REL 2016 Version 1.1. Available online: https://semmelweis.hu/gyermekklinika2/files/2022/05/ALL-IC-REL-2016_v1.1_2019-09-16.pdf (accessed on 10 September 2024).
- Locatelli, F.; Zugmaier, G.; Mergen, N.; Bader, P.; Jeha, S.; Schlegel, P.G.; Bourquin, J.P.; Handgretinger, R.; Brethon, B.; Rössig, C.; et al. Blinatumomab in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia: RIALTO expanded access study final analysis. Blood Adv. 2022, 6, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Zugmaier, G.; Rizzari, C.; Morris, J.D.; Gruhn, B.; Klingebiel, T.; Parasole, R.; Linderkamp, C.; Flotho, C.; Petit, A.; et al. Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children with High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2021, 325, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Hogan, L.E.; Borowitz, M.J.; Raetz, E.A.; Zugmaier, G.; Sharon, E.; Bernhardt, M.B.; et al. Effect of Postreinduction Therapy Consolidation with Blinatumomab vs Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults With First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2021, 325, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Messinger, Y.H.; Gaynon, P.S.; Sposto, R.; van der Giessen, J.; Eckroth, E.; Malvar, J.; Bostrom, B.C.; Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Consortium. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood 2012, 120, 285–290. [Google Scholar] [PubMed]
- Bertaina, A.; Vinti, L.; Strocchio, L.; Gaspari, S.; Caruso, R.; Algeri, M.; Coletti, V.; Gurnari, C.; Romano, M.; Cefalo, M.G.; et al. The combination of bortezomib with chemotherapy to treat relapsed/refractory acute lymphoblastic leukaemia of childhood. Br. J. Haematol. 2017, 176, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Mantadakis, E.; Stiakaki, E.; Groll, A.H.; Tragiannidis, A. Infectious Complications of Targeted Therapies in Children with Leukemias and Lymphomas. Cancers 2022, 14, 5022. [Google Scholar] [CrossRef] [PubMed]
- Raetz, E.A.; Cairo, M.S.; Borowitz, M.J.; Blaney, S.M.; Krailo, M.D.; Leil, T.A.; Reid, J.M.; Goldenberg, D.M.; Wegener, W.A.; Carroll, W.L.; et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: A Children’s Oncology Group Pilot Study. J. Clin. Oncol. 2008, 26, 3756–3762. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Stevenson, M.S.; Yuan, C.M.; Richards, K.; Delbrook, C.; Kreitman, R.J.; Pastan, I.; Wayne, A.S. Characterization of CD22 expression in acute lymphoblastic leukemia. Pediatr. Blood Cancer 2015, 62, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.M.; Jabbour, E.; Wang, T.; Liang White, J.; et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019, 125, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Bhojwani, D.; Sposto, R.; Shah, N.N.; Rodriguez, V.; Yuan, C.; Stetler-Stevenson, M.; O’Brien, M.M.; McNeer, J.L.; Quereshi, A.; Cabannes, A.; et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia 2019, 33, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; Cabannes-Hamy, A.; Adjaoud, D.; Bruno, B.; Blanc, L.; Boissel, N.; Tabone, M.D.; Willson-Plat, G.; Villemonteix, J.; Baruchel, A.; et al. Inotuzumab ozogamicin compassionate use for French paediatric patients with relapsed or refractory CD22-positive B-cell acute lymphoblastic leukaemia. Br. J. Haematol. 2020, 190, e53–e56. [Google Scholar] [CrossRef] [PubMed]
- Brivio, E.; Locatelli, F.; Lopez-Yurda, M.; Malone, A.; Díaz-de-Heredia, C.; Bielorai, B.; Rossig, C.; van der Velden, V.H.J.; Ammerlaan, A.C.J.; Thano, A.; et al. A phase 1 study of inotuzumab ozogamicin in pediatric relapsed/refractory acute lymphoblastic leukemia (ITCC-059 study). Blood 2021, 137, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Ogawa, C.; Sekimizu, M.; Fujisaki, H.; Kosaka, Y.; Hashimoto, H.; Saito, A.M.; Horibe, K. A phase I study of inotuzumab ozogamicin as a single agent in pediatric patients in Japan with relapsed/refractory CD22-positive acute lymphoblastic leukemia (INO-Ped-ALL-1). Int. J. Hematol. 2022, 116, 612–621. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.M.; Ji, L.; Shah, N.N.; Rheingold, S.R.; Bhojwani, D.; Yuan, C.M.; Xu, X.; Yi, J.S.; Harris, A.C.; Brown, P.A.; et al. Phase II Trial of Inotuzumab Ozogamicin in Children and Adolescents with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia: Children’s Oncology Group Protocol AALL1621. J. Clin. Oncol. 2022, 40, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, E.; Michels, N.; Brivio, E.; van der Velden, V.H.J.; Jiang, Y.; Thano, A.; Ammerlaan, A.J.C.; Boer, J.M.; Beverloo, H.B.; Sleight, B.; et al. Inotuzumab ozogamicin as single agent in pediatric patients with relapsed and refractory acute lymphoblastic leukemia: Results from a phase II trial. Leukemia 2022, 36, 1516–1524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tragiannidis, A.; Antari, V.; Tsotridou, E.; Sidiropoulos, T.; Kaisari, A.; Palabougiouki, M.; Vyzantiadis, T.-A.; Hatzipantelis, E.; Galli-Tsinopoulou, A.; Goussetis, E. Complete Remission with Inotuzumab Ozogamicin as Fourth-Line Salvage Therapy in a Child with Relapsed/Refractory Acute Lymphoblastic Leukemia. Hematol. Rep. 2024, 16, 579-584. https://doi.org/10.3390/hematolrep16040056
Tragiannidis A, Antari V, Tsotridou E, Sidiropoulos T, Kaisari A, Palabougiouki M, Vyzantiadis T-A, Hatzipantelis E, Galli-Tsinopoulou A, Goussetis E. Complete Remission with Inotuzumab Ozogamicin as Fourth-Line Salvage Therapy in a Child with Relapsed/Refractory Acute Lymphoblastic Leukemia. Hematology Reports. 2024; 16(4):579-584. https://doi.org/10.3390/hematolrep16040056
Chicago/Turabian StyleTragiannidis, Athanasios, Vassiliki Antari, Eleni Tsotridou, Theodoros Sidiropoulos, Aikaterini Kaisari, Maria Palabougiouki, Timoleon-Achilleas Vyzantiadis, Emmanuel Hatzipantelis, Assimina Galli-Tsinopoulou, and Evgenios Goussetis. 2024. "Complete Remission with Inotuzumab Ozogamicin as Fourth-Line Salvage Therapy in a Child with Relapsed/Refractory Acute Lymphoblastic Leukemia" Hematology Reports 16, no. 4: 579-584. https://doi.org/10.3390/hematolrep16040056
APA StyleTragiannidis, A., Antari, V., Tsotridou, E., Sidiropoulos, T., Kaisari, A., Palabougiouki, M., Vyzantiadis, T.-A., Hatzipantelis, E., Galli-Tsinopoulou, A., & Goussetis, E. (2024). Complete Remission with Inotuzumab Ozogamicin as Fourth-Line Salvage Therapy in a Child with Relapsed/Refractory Acute Lymphoblastic Leukemia. Hematology Reports, 16(4), 579-584. https://doi.org/10.3390/hematolrep16040056








