Goal-Directed Use of Prothrombin Complex Concentrates in Liver Transplantation: Is a Plasma-Free Procedure Feasible?
Abstract
1. Introduction
2. PCC Characteristics
3. Rationale for PCC Use in LT Patients
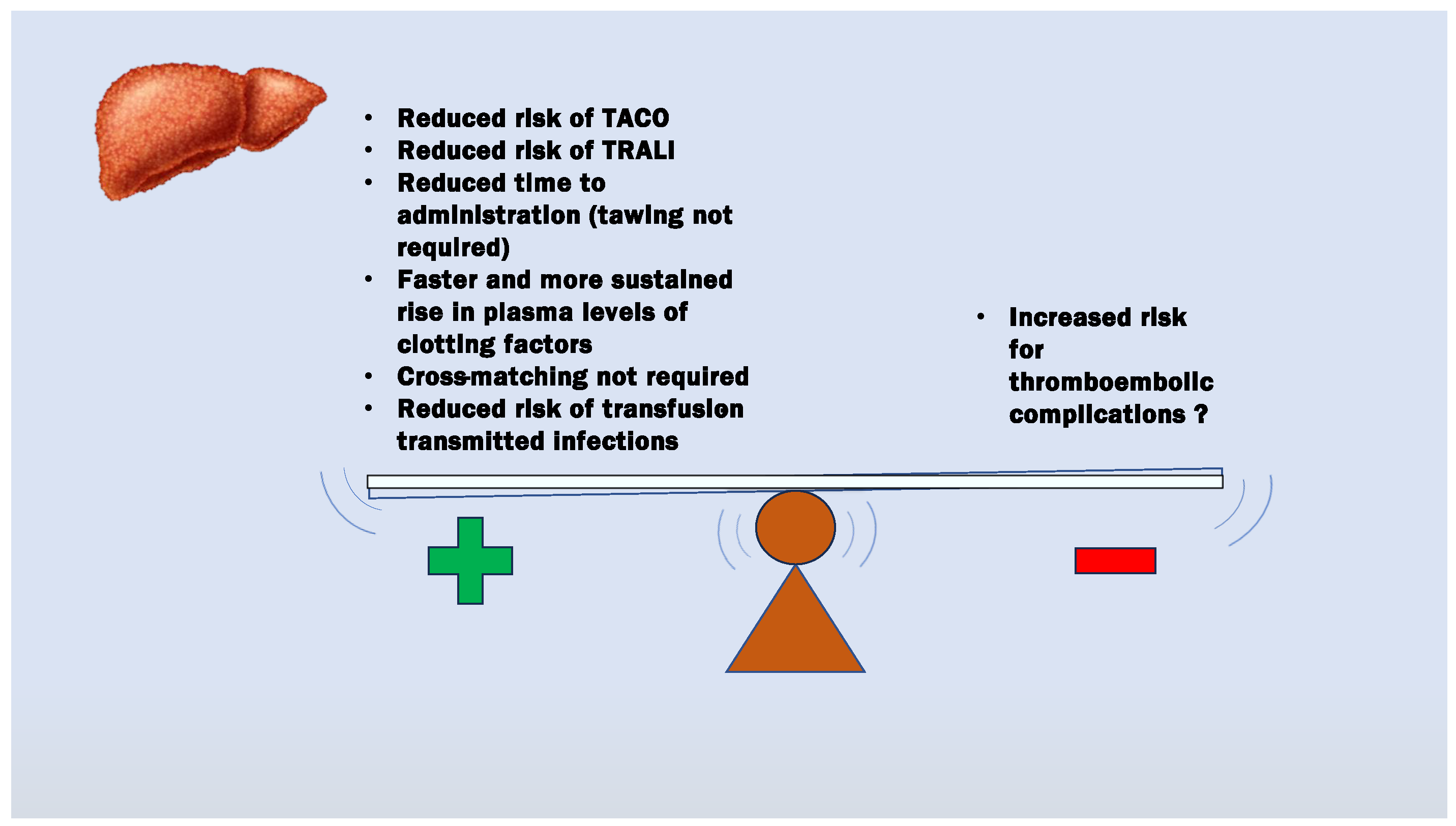
4. Evidence of Benefits from Coagulation Factor Concentrate Therapy in LT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Görlinger, K.; Pérez-Ferrer, A.; Dirkmann, D.; Saner, F.; Maegele, M.; Calatayud, Á.A.P.; Kim, T.Y. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J. Anesthesiol. 2019, 72, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Fries, D.; Dirkmann, D.; Weber, C.F.; Hanke, A.A.; Schöchl, H. Reduction of Fresh Frozen Plasma Requirements by Perioperative Point-of-Care Coagulation Management with Early Calculated Goal-Directed Therapy. Transfus. Med. Hemother. 2012, 39, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Dehne, S.; Riede, C.; Klotz, R.; Sander, A.; Feisst, M.; Merle, U.; Mieth, M.; Golriz, M.; Mehrabi, A.; Büchler, M.W.; et al. Perioperative prothrombin complex concentrate and fibrinogen administration are associated with thrombotic complications after liver transplant. Front. Med. 2022, 9, 1043674. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, C.; Dirkmann, D.; Treckmann, J.W.; Paul, A.; Hartmann, M.; Saner, F.H.; Görlinger, K. Coagulation management with factor concentrates in liver transplantation: A single-center experience. Transfusion 2014, 54 Pt 2, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Colavecchia, A.C.; Cohen, D.A.; Harris, J.E.; Thomas, J.M.; Lindberg, S.; Leveque, C.; Salazar, E. Impact of intraoperative factor concentrates on blood product transfusions during orthotopic liver transplantation. Transfusion 2017, 57, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Agarwal, A.; Jha, A.; Rodricks, S.; Malik, T.; Makki, K.; Singhal, A.; Vij, V. Utility of prothrombin complex concentrate as first-line treatment modality of coagulopathy in patients undergoing liver transplantation: A propensity score-matched study. Clin. Transplant. 2018, 32, e13435. [Google Scholar] [CrossRef] [PubMed]
- Zamper, R.P.C.; Amorim, T.C.; Queiroz, V.N.F.; Lira, J.D.O.; Costa, L.G.V.; Takaoka, F.; Juffermans, N.P.; Neto, A.S. Association between viscoelastic tests-guided therapy with synthetic factor concentrates and allogenic blood transfusion in liver transplantation: A before-after study. BMC Anesthesiol. 2018, 18, 198. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Walde, C.; Dirkmann, D.; Saner, F.H. Safety of coagulation factor concentrates guided by ROTEM™-analyses in liver transplantation: Results from 372 procedures. BMC Anesthesiol. 2019, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Punzo, G.; Di Franco, V.; Perilli, V.; Sacco, T.; Sollazzi, L.; Aceto, P. Efficacy and Safety of Prothrombin Complex Concentrates in Liver Transplantation: Evidence from Observational Studies. J. Clin. Med. 2023, 12, 3749. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, L.; Lenis, S.; Thibeault, L.; Sassine, M.P.; Seal, R.F.; Roy, A. Effect of low central venous pressure and phlebotomy on blood product transfusion requirements during liver transplantations. Liver Transpl. 2006, 12, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Refaai, M.A.; Goldstein, J.N.; Lee, M.L.; Durn, B.L.; Milling, T.J., Jr.; Sarode, R. Increased risk of volume overload with plasma compared with four-factor prothrombin complex concentrate for urgent vitamin K antagonist reversal. Transfusion 2015, 55, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K. Gerinnungsmanagement bei Lebertransplantationen [Coagulation management during liver transplantation]. Hamostaseologie 2006, 26 (Suppl. S1), S64–S76. (In German) [Google Scholar] [PubMed]
- Tischendorf, M.; Fuchs, A.; Zeuzem, S.; Lange, C.M. Use of prothrombin complex concentrates in patients with decompensated liver cirrhosis is associated with thromboembolic events. J. Hepatol. 2019, 70, 800–801. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, T.; Kelly, G.; Kersten, B.; Nadler, M.; Zammit, C.G.; Jones, C.M.C.; Scott, R.; Acquisto, N.M. Incidence of thromboembolic events following administration of four-factor prothrombin complex concentrate (4F-PCC) for oral anticoagulation reversal. Thromb. Res. 2020, 194, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 3rd ed.; Council of Europe: Strasbourg, France, 1996; Volume 8, pp. 29–30. [Google Scholar]
- Erdoes, G.; Koster, A.; Ortmann, E.; Meesters, M.I.; Bolliger, D.; Baryshnikova, E.; Martinez Lopez De Arroyabe, B.; Ahmed, A.; Lance, M.D.; Ranucci, M.; et al. A European Consensus Statement on the Use of Four-Factor Prothrombin Complex Concentrate for Cardiac and Non-Cardiac Surgical Patients. Anaesthesia 2021, 76, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.J.; Hira, S.K.; Sison, M.L.; Getrajdman, C.S. Impact of fibrinogen and prothrombin complex concentrate on clotting time in a model of obstetric hemorrhage. J. Clin. Anesth. 2022, 78, 110687. [Google Scholar] [CrossRef] [PubMed]
- Biancofiore, G.; Blasi, A.; De Boer, M.T.; Franchini, M.; Hartmann, M.; Lisman, T.; Liumbruno, G.M.; Porte, R.J.; Saner, F.; Senzolo, M.; et al. Perioperative Hemostatic Management in the Cirrhotic Patient: A Position Paper on behalf of the Liver Intensive Care Group of Europe (LICAGE). Minerva Anestesiol. 2019, 85, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Teofili, L.; Valentini, C.G.; Aceto, P.; Bartolo, M.; Sollazzi, L.; Agnes, S.; Gaspari, R.; Avolio, A.W. High intraoperative blood product requirements in liver transplantation: Risk factors and impact on the outcome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Stanworth, S.J.; Brunskill, S.J.; Hyde, C.J.; McClelland, D.B.; Murphy, M.F. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br. J. Haematol. 2004, 126, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, R.; Teofili, L.; Aceto, P.; Valentini, C.G.; Punzo, G.; Sollazzi, L.; Agnes, S.; Avolio, A.W. Thromboelastography does not reduce transfusion requirements in liver transplantation: A propensity score-matched study. J. Clin. Anesth. 2021, 69, 110154. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.K.; Kim, S.; Hill, B.; Goldberg, A.; DeMaria, S.; Zerillo, J. Transfusion-Related Acute Lung Injury (TRALI) and Transfusion-Associated Circulatory Overload (TACO) in Liver Transplantation: A Case Report and Focused Review. Semin. Cardiothorac. Vasc. Anesth. 2018, 22, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kuldanek, S.A.; Kelher, M.; Silliman, C.C. Risk factors, management and prevention of transfusion-related acute lung injury: A comprehensive update. Expert. Rev. Hematol. 2019, 12, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.H.; Kumar, J.E.; Kumar, N.; Gorelik, L.; Hussain, N.; Stein, E.; Bhatt, A.M.; Bhandary, S.; Essandoh, M.K.; Flores, A.S. Transfusion-Related Acute Lung Injury During Liver Transplantation: A Scoping Review. J. Cardiothorac. Vasc. Anesth. 2022, 36 Pt A, 2606–2615. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.G.; Greenberg, C.S.; Patton, H.M.; Caldwell, S.H. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology 2019, 157, 34–43.e1. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Cang, W.C.; Derry, K.L.; Lane, J.R.; von Drygalski, A. Four-Factor Prothrombin Complex Concentrate for Coagulopathy Reversal in Patients with Liver Disease. Clin. Appl. Thromb. Hemost. 2017, 23, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Hellstern, P. Production and composition of prothrombin complex concentrates: Correlation between composition and therapeutic efficiency. Thromb. Res. 1999, 95 (Suppl. S1), S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Kalina, U.; Bickhard, H.; Schulte, S. Biochemical comparison of seven commercially available prothrombin complex concentrates. Int. J. Clin. Pract. 2008, 62, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Kahn, D.; Sayed, D.; Hoppenstadt, D.; Jeske, W.; Harenberg, J.; Dechristopher, P.; Fareed, J. Compositional differences in commercially available prothrombin complex concentrates. Clin. Appl. Thromb. Hemost. 2014, 20, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; van den Boom, B.; Ow, T.W.; Prachalias, A.; Adelmeijer, J.; Phoolchund, A.; Dunsire, F.; Milan, Z.; Roest, M.; Heaton, N.; et al. Efficacy of pro- and anticoagulant strategies in plasma of patients undergoing hepatobiliary surgery. J. Thromb. Haemost. 2020, 18, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Abuelkasem, E.; Hasan, S.; Mazzeffi, M.A.; Planinsic, R.M.; Sakai, T.; Tanaka, K.A. Reduced Requirement for Prothrombin Complex Concentrate for the Restoration of Thrombin Generation in Plasma from Liver Transplant Recipients. Anesth. Analg. 2017, 125, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, D.P.; Wirtz, M.R.; Neto, A.S.; Schöchl, H.; Viersen, V.; Binnekade, J.; Juffermans, N.P. Effectiveness of prothrombin complex concentrate for the treatment of bleeding: A systematic review and meta-analysis. J. Thromb. Haemost. 2020, 18, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Gazzard, B.G.; Henderson, J.M.; Williams, R. The use of fresh frozen plasma or a concentrate of factor IX as replacement therapy before liver biopsy. Gut 1975, 16, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M.; Franchi, F.; Dioguardi, N. Correction of abnormal coagulation in chronic liver disease by combined use of fresh-frozen plasma and prothrombin complex concentrates. Lancet 1976, 2, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Gille, A.; Erhard, J.; Paar, D.; Kox, W.J. Substitutionseffekt von AT III-und PPSB-Konzentraten bei Patienten mit terminaler Leberinsuffizienz [The effect of substitution with AT III- and PPSB-concentrates in patients with terminal liver insufficiency]. Anaesthesist 1994, 43, 178–182. (In German) [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Ickx, B.; van Beem, R.T.; Polak, W.; Grüne, F.; Nevens, F.; Ilmakunnas, M.; Koivusalo, A.M.; Isoniemi, H. , Strengers, P.F.; et al. Prothrombin complex concentrate in the reduction of blood loss during orthotopic liver transplantation: PROTON-trial. BMC Surg. 2013, 13, 22. [Google Scholar] [CrossRef] [PubMed]
| PCC (Manufacturer) | Factors (IU/mL) | Coagulation Proteins (IU/mL) | Other Antithrombotic Additions | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F. II | F. VII | F. IX | F. X | Protein S | Protein C | Protein Z | |||
| 3F-PCC | Bebulin® (Baxter Healthcare Corporation, Westlake village, CA, USA) | 24–37 | <5 | 24–37 | 24–37 | N/A | N/A | No | Small amounts of heparin (<0.15 IU per IU F.IX) |
| Profilnine® (Grifols Biologicals, Barcelona, Spain) | 87 | - | 69 | 54 | 0 | 0 | No | None | |
| UMAN Complex D.I. (Kedrion, Italy) | 28 | <0.1 | 28 | 21 | 5 | 9 | Yes | Antithrombin, heparin | |
| 4F-PCC | Beriplex®/Confidex®/Kcentra® (CSL Behring, Marburg, Germany) | 20–48 | 10–25 | 20–31 | 22–60 | 17–19 | 22–31 | Yes | Antithrombin, heparin, albumin |
| Cofact® (Sanquin, Amsterdam, The Netherlands) | 30 | 13 | 23 | 26 | 21 | 4 | Yes | Antithrombin | |
| Octaplex® (Octapharma, Brussels, Belgium) | 31 | 16 | 22 | 24 | 24 | 12 | Yes | Heparin, low activated Factor VIIa | |
| Prothromplex T® (Baxter Bioscence, Vienna, Austria) | 12 | 11 | 8 | 11 | 8 | 4 | Yes | Antithrombin, heparin | |
| Author (Year) | Study Type | Patients | PCC | 3F-/4F-PCC | Mean Dose PCC | Coagulation Monitoring | Clinical Outcome | Incidence of Thrombotic and/or Thromboembolic Events (FC Group vs. the Non-FC Group) |
|---|---|---|---|---|---|---|---|---|
| Kirchner (2014) [4] | Retrospective study | 266 (FC group n = 156) | Beriplex® | 4F-PCC | 4090 ± 3130 IU | ROTEM | Incidence of thrombotic, thromboembolic and ischemic events in the first 10 postoperative days | 7.1% vs. 4.5% p = 0.31 |
| Colavecchia (2017) [5] | Retrospective/ Propensity-matched | 117 * (PCC group n = 39) | Kcentra® | 4F-PCC | 902 ± 589 IU | SCT | Reduction in allogenic blood product use | N/A |
| Srivastava (2018) [6] | Retrospective/Propensity-matched | 120 * (PCC group n = 60) | N/A | N/A | N/A. 25 IU/kg given if TEG R > 10 min. | TEG | Reduction in allogenic blood product use | 0.0% vs. 0.0% |
| Zamper (2018) [7] | Retrospective/Propensity-matched | 135 * (PCC group n = 46) | N/A | N/A | 195.6 ± 645.3 IU | ROTEM | Reduction in allogenic blood product use | N/A |
| Hartman (2019) [8] | Retrospective study | 372 (PCC group n = 70) | Kcentra® | 4F-PCC | N/A. Range 1000–7000 IU | ROTEM | Reduction in 30-day mortality in LT | N/A |
| Dehne (2022) [3] | Retrospective study | 939 (FC group n = 576) | N/A | N/A | 3350 ± 3500 IU | TEG or ROTEM | Occurrence of hepatic artery, portal vein or inferior vena cava thrombosis within the first 30 days after surgery | 11.3% vs. 6.6% p = 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punzo, G.; Di Franco, V.; Aceto, P. Goal-Directed Use of Prothrombin Complex Concentrates in Liver Transplantation: Is a Plasma-Free Procedure Feasible? Hematol. Rep. 2024, 16, 454-464. https://doi.org/10.3390/hematolrep16030044
Punzo G, Di Franco V, Aceto P. Goal-Directed Use of Prothrombin Complex Concentrates in Liver Transplantation: Is a Plasma-Free Procedure Feasible? Hematology Reports. 2024; 16(3):454-464. https://doi.org/10.3390/hematolrep16030044
Chicago/Turabian StylePunzo, Giovanni, Valeria Di Franco, and Paola Aceto. 2024. "Goal-Directed Use of Prothrombin Complex Concentrates in Liver Transplantation: Is a Plasma-Free Procedure Feasible?" Hematology Reports 16, no. 3: 454-464. https://doi.org/10.3390/hematolrep16030044
APA StylePunzo, G., Di Franco, V., & Aceto, P. (2024). Goal-Directed Use of Prothrombin Complex Concentrates in Liver Transplantation: Is a Plasma-Free Procedure Feasible? Hematology Reports, 16(3), 454-464. https://doi.org/10.3390/hematolrep16030044






