Treatment Patterns and Clinical Outcomes of Patients with Moderate to Severe Acute Graft-Versus-Host Disease: A Multicenter Chart Review Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Ethical Considerations
2.4. Statistical Analyses
3. Results
3.1. Patients
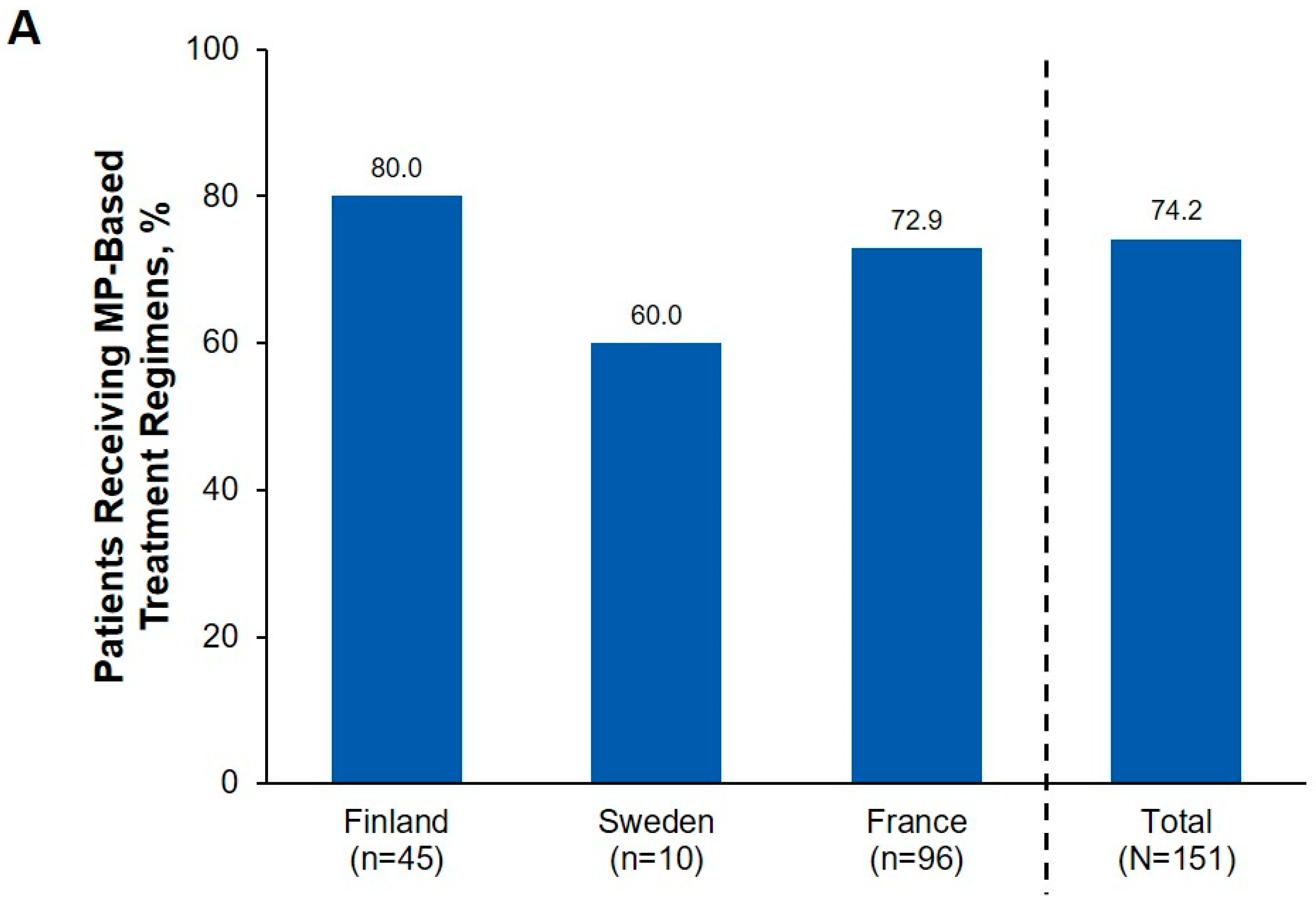
3.2. Treatment Patterns
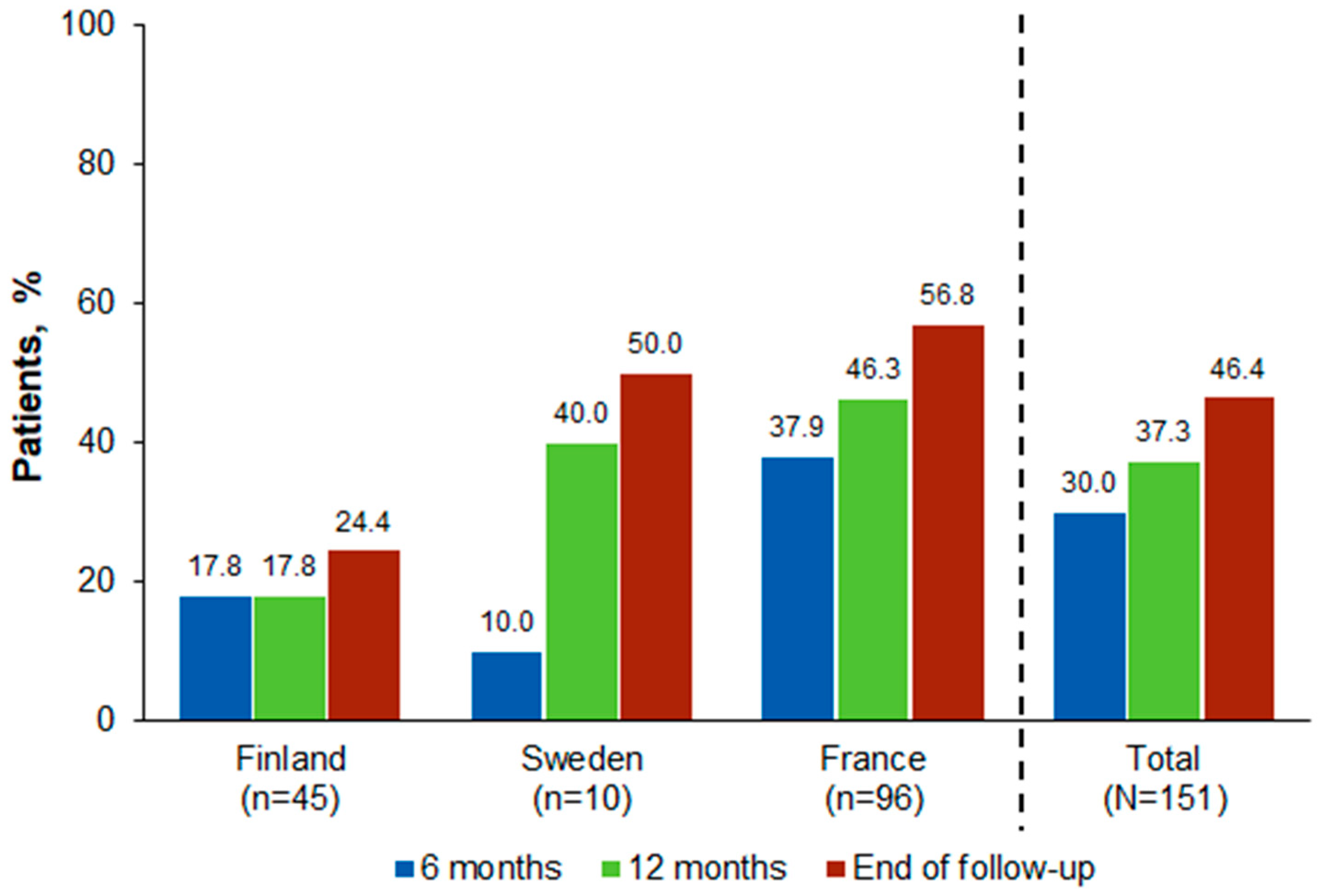
3.3. Clinical Outcomes
3.4. Subgroup Analysis
3.5. All-Cause Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, R.F.; Labopin, M.; Bader, P.; Basak, G.W.; Bonini, C.; Chabannon, C.; Corbacioglu, S.; Dreger, P.; Dufour, C.; Gennery, A.R.; et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2019. Bone Marrow Transpl. 2019, 54, 1525–1552. [Google Scholar] [CrossRef] [PubMed]
- Passweg, J.R.; Baldomero, H.; Basak, G.W.; Chabannon, C.; Corbacioglu, S.; Duarte, R.; Kuball, J.; Lankester, A.; Montoto, S.; de Latour, R.P.; et al. The EBMT activity survey report 2017: A focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transpl. 2019, 54, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Magenau, J.; Runaas, L.; Reddy, P. Advances in understanding the pathogenesis of graft-versus-host disease. Br. J. Haematol. 2016, 173, 190–205. [Google Scholar] [CrossRef]
- Axt, L.; Naumann, A.; Toennies, J.; Haen, S.P.; Vogel, W.; Schneidawind, D.; Wirths, S.; Moehle, R.; Faul, C.; Kanz, L.; et al. Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2019, 54, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Lorentino, F.; Nitti, R.; Lupo Stanghellini, M.T.; Giglio, F.; Clerici, D.; Xue, E.; Lazzari, L.; Piemontese, S.; Mastaglio, S.; et al. Interleukin-6 as biomarker for acute GvHD and survival after allogeneic transplant with post-transplant cyclophosphamide. Front. Immunol. 2019, 10, 2319. [Google Scholar] [CrossRef] [PubMed]
- Al Malki, M.M.; Gendzekhadze, K.; Yang, D.; Mokhtari, S.; Parker, P.; Karanes, C.; Palmer, J.; Snyder, D.; Forman, S.J.; Nademanee, A.; et al. Long-term outcome of allogeneic hematopoietic stem cell transplantation from unrelated donor using tacrolimus/sirolimus-based GVHD prophylaxis: Impact of HLA mismatch. Transplantation 2020, 104, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Holler, E.; Sandmaier, B.M.; Huang, H.; Mohty, M. Acute graft-versus-host disease. Nat. Rev. Dis. Primers 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Holtan, S.G.; DeFor, T.E.; Lazaryan, A.; Bejanyan, N.; Arora, M.; Brunstein, C.G.; Blazar, B.R.; MacMillan, M.L.; Weisdorf, D.J. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015, 125, 1333–1338. [Google Scholar] [CrossRef]
- Bazarbachi, A.H.; Al Hamed, R.; Labopin, M.; Afanasyev, B.; Hamladji, R.M.; Beelen, D.; Eder, M.; Scheid, C.; Wu, D.; Bunjes, D.; et al. Allogeneic stem-cell transplantation with sequential conditioning in adult patients with refractory or relapsed acute lymphoblastic leukemia: A report from the EBMT Acute Leukemia Working Party. Bone Marrow Transpl. 2020, 55, 595–602. [Google Scholar] [CrossRef]
- Westin, J.R.; Saliba, R.M.; De Lima, M.; Alousi, A.; Hosing, C.; Qazilbash, M.H.; Khouri, I.F.; Shpall, E.J.; Anderlini, P.; Rondon, G.; et al. Steroid-refractory acute GVHD: Predictors and outcomes. Adv. Hematol. 2011, 2011, 601953. [Google Scholar] [CrossRef] [PubMed]
- Major-Monfried, H.; Renteria, A.S.; Pawarode, A.; Reddy, P.; Ayuk, F.; Holler, E.; Efebera, Y.A.; Hogan, W.J.; Wolfl, M.; Qayed, M.; et al. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood 2018, 131, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Drahos, J.; Johnson, B.H.; Kim, G.; Yang, J.; Taylor, A. Use of real-world evidence to characterize clinical outcomes in patients who develop acute graft versus host disease undergoing allogeneic stem cell transplantation in the United States. Biol. Blood Marrow Transplant. 2018, 24, S308–S309. [Google Scholar] [CrossRef]
- Yu, J.; Judy, J.T.; Parasuraman, S.; Sinha, M.; Weisdorf, D. Inpatient healthcare resource utilization, costs, and mortality in adult patients with acute graft-versus-host disease, including steroid-refractory or high-risk disease, following allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 2020, 26, 600–605. [Google Scholar] [CrossRef]
- Johnson, B.H.; Taylor, A.; Kim, G.; Drahos, J.; Yang, J.; Akbari, M.; Shah, N.N. Clinical outcomes and healthcare resource utilization for gastrointestinal acute graft-versus-host disease after allogeneic transplantation for hematologic malignancy: A retrospective US administrative claims database analysis. Biol. Blood Marrow Transpl. 2019, 25, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Khoury, H.J.; Wang, T.; Hemmer, M.T.; Couriel, D.; Alousi, A.; Cutler, C.; Aljurf, M.; Antin, J.H.; Ayas, M.; Battiwalla, M.; et al. Improved survival after acute graft-versus-host disease diagnosis in the modern era. Haematologica 2017, 102, 958–966. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.B.; Sandmaier, B.M.; Mielcarek, M.; Sorror, M.; Pergam, S.A.; Cheng, G.S.; Hingorani, S.; Boeckh, M.; Flowers, M.D.; Lee, S.J.; et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: Comparing 2003–2007 versus 2013–2017 cohorts. Ann. Intern. Med. 2020, 172, 229–239. [Google Scholar] [CrossRef]
- Sabatelli, L.; Keränen, M.; Viayna, E.; Roset, M.; Lara, N.; Thunström, D.; Pfeiffer, M.; Nicklasson, M.; Itälä-Remes, M. Burden of hospitalizations and outpatient visits associated with moderate and severe acute graft-versus-host disease in Finland and Sweden: A real-world data study. Support. Care Cancer 2022, 30, 5125–5135. [Google Scholar] [CrossRef]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: A report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transpl. 2016, 22, 4–10. [Google Scholar] [CrossRef]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995, 15, 825–828. [Google Scholar]
- Rowlings, P.A.; Przepiorka, D.; Klein, J.P.; Gale, R.P.; Passweg, J.R.; Henslee-Downey, P.J.; Cahn, J.Y.; Calderwood, S.; Gratwohl, A.; Socie, G.; et al. IBMTR Severity Index for grading acute graft-versus-host disease: Retrospective comparison with Glucksberg grade. Br. J. Haematol. 1997, 97, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Schoemans, H.M.; Lee, S.J.; Ferrara, J.L.; Wolff, D.; Levine, J.E.; Schultz, K.R.; Shaw, B.E.; Flowers, M.E.; Ruutu, T.; Greinix, H.; et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transpl. 2018, 53, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Holtan, S.G.; Yu, J.; Choe, H.K.; Paranagama, D.; Tang, J.; Naim, A.; Galvin, J.; Joachim Deeg, H. Disease progression, treatments, hospitalization, and clinical outcomes in acute GVHD: A multicenter chart review. Bone Marrow Transpl. 2022, 57, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Finland (n = 2) | Sweden (n = 1) | France (n = 10) | Total (n = 13) |
|---|---|---|---|---|
| Type of transplant center, n (%) 1 | ||||
| Academic/university | 2 (100.0) | 1 (100.0) | 8 (80.0) | 11 (84.6) |
| Public | 1 (50.0) | 0 | 6 (60.0) | 7 (53.8) |
| Specialized cancer center | 0 | 0 | 1 (10.0) | 1 (7.7) |
| Physicians dedicated to HSCT (≥90% of the time), median (Q1, Q3), n | 3.5 (3.0, 4.0) | 3.0 (3.0, 3.0) | 4.5 (3.0, 7.0) | 4.0 (3.0, 5.0) |
| Adult HSCTs conducted (1 January 2016–30 June 2017), median (Q1, Q3), n | 95.5 (76.0, 115.0) | 53.0 (53.0, 53.0) | 94.0 (70.0, 120.0) | 91.0 (70.0, 115.0) |
| Criteria used to grade aGVHD, n (%) 1 | ||||
| MAGIC | 1 (50.0) | 0 | 2 (20.0) | 3 (23.1) |
| Original Glucksberg | 0 | 0 | 6 (60.0) | 6 (46.2) |
| Modified Glucksberg or Keystone | 1 (50.0) | 1 (100.0) | 5 (50.0) | 7 (53.8) |
| IBMTR | 0 | 0 | 1 (10.0) | 1 (7.7) |
| Other | 0 | 0 | 1 (10.0) | 1 (7.7) |
| Patients with severe (grades III/IV) aGVHD after allogeneic HSCT, median (Q1, Q3), % | 34.3 (18.5, 50.0) | 25.0 (25.0, 25.0) | 30.0 (30.0, 50.0) | 30.0 (30.0, 50.0) |
| Characteristic | Finland (n = 45) | Sweden (n = 10) | France (n = 96) | Total (n = 151) |
|---|---|---|---|---|
| Sex, n (%) 1 | ||||
| Male | 25 (55.6) | 5 (50.0) | 65 (67.7) | 95 (62.9) |
| Female | 20 (44.4) | 5 (50.0) | 30 (31.3) | 55 (36.4) |
| Age at transplant, median (Q1, Q3), y 1 | 54.0 (45.0, 59.0) | 44.5 (36.0, 61.0) | 56.0 (46.0, 65.0) | 55.5 (45.0, 62.0) |
| Age at grade II–IV aGVHD diagnosis, median (Q1, Q3), y | 54.0 (45.0, 59.0) | 44.5 (36.0, 61.0) | 56.0 (46.0, 65.0) | 55.5 (45.0, 62.0) |
| BMI, median (Q1, Q3), kg/m 2,3 | 27.8 (23.4, 29.6) | 25.4 (24.5, 26.5) | 25.0 (22.1, 27.2) | 25.4 (22.7, 27.9) |
| Primary disease diagnosis, n (%) | ||||
| AML | 16 (35.6) | 3 (30.0) | 33 (34.4) | 52 (34.4) |
| ALL | 3 (6.7) | 1 (10.0) | 19 (19.8) | 23 (15.2) |
| MDS | 4 (8.9) | 0 | 11 (11.5) | 15 (9.9) |
| MM | 8 (17.8) | 0 | 1 (1.0) | 9 (6.0) |
| Hodgkin lymphoma | 3 (6.7) | 1 (10.0) | 4 (4.2) | 8 (5.3) |
| B-cell lymphoma | 3 (6.7) | 2 (20.0) | 0 | 5 (3.3) |
| Non–CML MPN | 4 (8.9) | 1 (10.0) | 0 | 5 (3.3) |
| CML | 1 (2.2) | 0 | 2 (2.1) | 3 (2.0) |
| T-cell lymphoma | 0 | 1 (10.0) | 0 | 1 (0.7) |
| Other malignancy 2 | 3 (6.7) | 1 (10.0) | 25 (26.0) | 29 (19.2) |
| Unknown | 0 | 0 | 1 (1.0) | 1 (0.7) |
| Stage at transplant, n (%) | ||||
| Complete remission | 29 (64.4) | 5 (50.0) | 59 (61.5) | 93 (61.6) |
| Partial remission | 9 (20.0) | 3 (30.0) | 5 (5.2) | 17 (11.3) |
| Active relapse or progressive disease | 5 (11.1) | 1 (10.0) | 15 (15.6) | 21 (13.9) |
| Patient untreated | 2 (4.4) | 1 (10.0) | 0 | 3 (2.0) |
| Unknown/other | 0 | 0 | 17 (17.7) | 17 (11.3) |
| Disease risk index, n (%) | ||||
| Low | 8 (17.8) | 2 (20.0) | 4 (4.2) | 14 (9.3) |
| Intermediate | 21 (46.7) | 6 (60.0) | 17 (17.7) | 44 (29.1) |
| High | 9 (20.0) | 2 (20.0) | 4 (4.2) | 15 (9.9) |
| Very high | 1 (2.2) | 0 | 1 (1.0) | 2 (1.3) |
| Unknown | 6 (13.3) | 0 | 70 (72.9) | 76 (50.3) |
| Conditioning regimen, n (%) | ||||
| Fludarabine-based | 32 (71.1) | 9 (90.0) | 66 (68.8) | 107 (70.9) |
| Busulfan-based | 7 (15.6) | 4 (40.0) | 54 (56.3) | 65 (43.0) |
| TBI-based | 14 (31.1) | 1 (10.0) | 23 (24.0) | 38 (25.2) |
| Other | 3 (6.7) | 7 (70.0) | 73 (76.0) | 83 (55.0) |
| Donor type, n (%) | ||||
| Related donor | 8 (17.8) | 5 (50.0) | 44 (45.8) | 57 (37.7) |
| Fully HLA-matched twin | 5 (62.5) | 0 | 0 | 5 (8.8) |
| Haploidentical donor | 0 | 1 (20.0) | 17 (38.6) | 18 (31.6) |
| HLA-matched related donor | 3 (37.5) | 4 (80.0) | 27 (61.4) | 34 (59.6) |
| Unrelated donor | 37 (82.2) | 5 (50.0) | 51 (53.1) | 93 (61.6) |
| HLA matched | 35 (94.6) | 5 (100.0) | 43 (84.3) | 83 (89.2) |
| HLA mismatched | 2 (5.4) | 0 | 7 (13.7) | 9 (9.7) |
| Unknown | 0 | 0 | 1 (1.0) | 1 (0.7) |
| Characteristic | Finland (n = 45) | Sweden (n = 10) | France (n = 96) | Total (n = 151) | ||||
|---|---|---|---|---|---|---|---|---|
| Number of treatment lines | ||||||||
| Median (Q1, Q3) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 1.0 (1.0–3.0) 1 | 2.0 (1.0–3.0) | ||||
| Distribution, n (%) | ||||||||
| 1 | 20 (44.4) | 4 (40.0) | 48 (50.0) | 72 (47.7) | ||||
| 2 | 13 (28.9) | 3 (30.0) | 20 (20.8) | 36 (23.8) | ||||
| 3 | 7 (15.6) | 1 (10.0) | 15 (15.6) | 23 (15.2) | ||||
| 4 | 3 (6.7) | 1 (10.0) | 4 (4.2) | 8 (5.3) | ||||
| 5 | 1 (2.2) | 1 (10.0) | 4 (4.2) | 6 (4.0) | ||||
| ≥6 | 1 (2.2) | 0 | 3 (3.1) | 4 (2.6) | ||||
| Unknown | 0 | 0 | 2 (2.1) | 2 (1.3) | ||||
| Duration of treatment, median (Q1, Q3), d | n | n | n | n | ||||
| First line | 25 | 6.0 (5.0, 18.0) | 6 | 25.5 (8.0, 87.0) | 20 | 17.5 (4.5, 60.5) | 51 | 9.0 (5.0, 40.0) |
| Second line | 12 | 19.5 (12.5, 33.5) | 3 | 20.0 (8.0, 102.0) | 7 | 27.0 (4.0, 72.0) | 22 | 21.0 (12.0, 38.0) |
| Third line | 5 | 31.0 (19.0, 79.0) | 2 | 78.0 (48.0, 108.0) | 3 | 30.0 (11.0, 60.0) | 10 | 39.5 (19.0, 79.0) |
| Fourth line | 2 | 59.0 (8.0, 110.0) | 1 | 306.0 (306.0, 306.0) | 3 | 36.0 (9.0, 40.0) | 6 | 38.0 (9.0, 110.0) |
| Fifth line | 1 | 211.0 (211.0, 211.0) | 0 | N/A | 1 | 88.0 (88.0, 88.0) | 2 | 149.5 (88.0–211.0) |
| Total duration of therapy, median (Q1, Q3), d | 40 | 169.5 (86.0, 260.0) | 6 | 196.0 (153.0, 227.0) | 43 | 86.0 (45.0, 169.0) | 89 | 131.0 (71.0, 208.0) |
| Treatment response of CR, VGPR, or PR, n (%) 2 | n | n | n | n | ||||
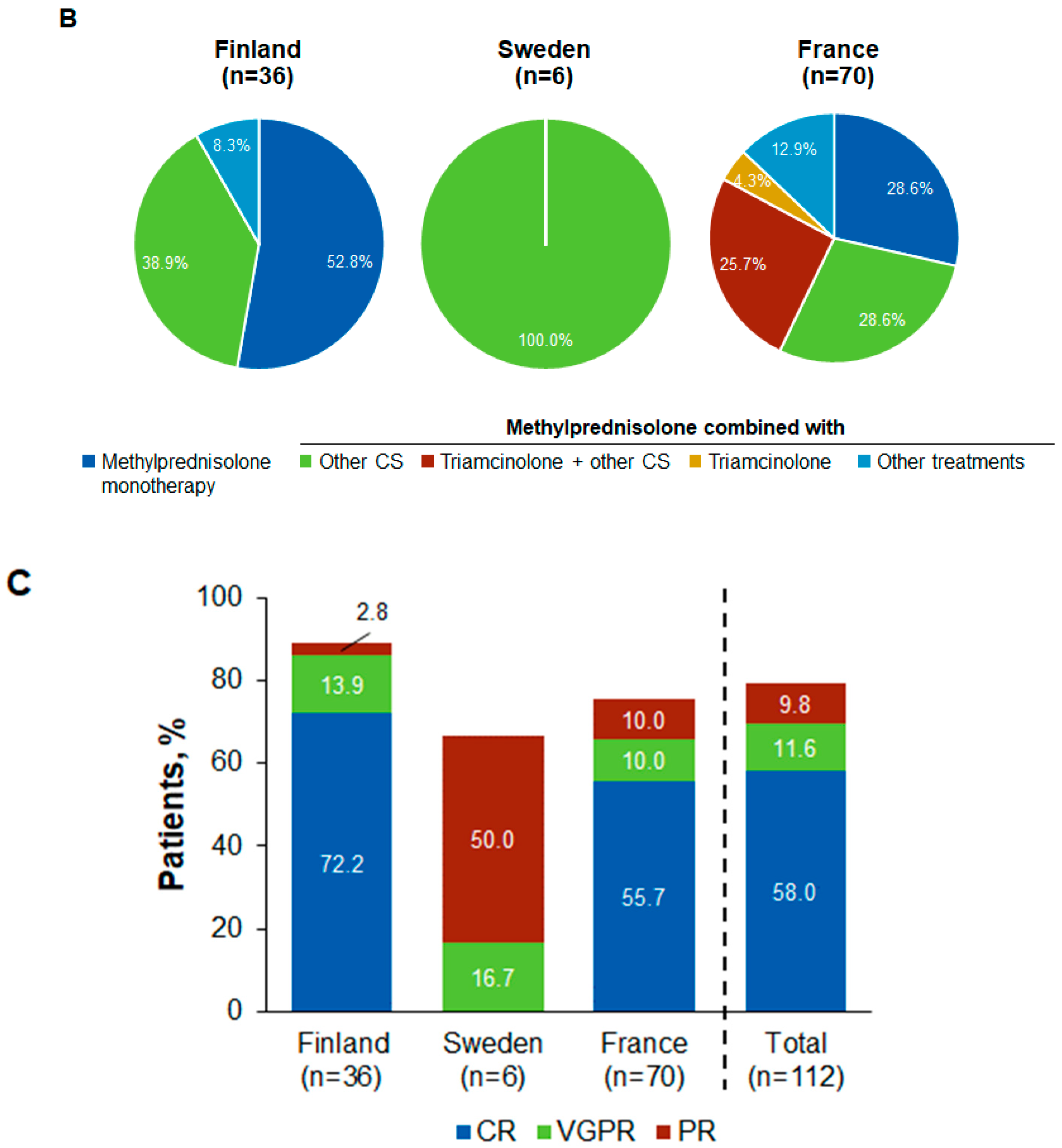
| Methylprednisolone-based therapy | 36 | 32 (88.9) | 6 | 4 (66.7) | 70 | 53 (75.7) | 112 | 89 (79.5) |
| Triamcinolone-based therapy | 0 | – | 0 | – | 40 | 36 (90.0) | 40 | 36 (90.0) |
| Other corticosteroids | 21 | 19 (90.5) | 10 | 8 (80.0) | 64 | 56 (87.5) | 95 | 83 (87.4) |
| Outcome | Cyclosporine + Mycophenolate (n = 40) | In Vivo T-Cell Depletion + Cyclosporine + Methotrexate (n = 15) | In Vivo T-Cell Depletion + Cyclosporine + Mycophenolate (n = 16) | In Vivo T-Cell Depletion + Methotrexate + Mycophenolate (n = 25) | Methotrexate + Mycophenolate (n = 18) | Mycophenolate (n = 13) |
|---|---|---|---|---|---|---|
| Time from HSCT to aGVHD diagnosis | ||||||
| Median (Q1, Q3), d | 28.0 (17.5, 35.0) | 57.0 (33.0, 76.0) | 23.0 (15.0, 36.0) | 34.0 (24.0, 44.0) | 25.5 (20.0, 40.0) | 30.0 (27.0, 49.0) |
| Best overall response, n (%) | ||||||
| 6 months | ||||||
| CR | 17 (42.5) | 11 (73.3) | 7 (43.8) | 16 (64.0) | 8 (44.4) | 7 (53.8) |
| VGPR | 0 | 2 (13.3) | 1 (6.3) | 1 (4.0) | 1 (5.6) | 0 |
| PR | 3 (7.5) | 1 (6.7) | 1 (6.3) | 1 (4.0) | 1 (5.6) | 1 (7.7) |
| Mixed disease | 0 | 0 | 0 | 1 (4.0) | 1 (5.6) | 0 |
| PD | 0 | 0 | 1 (6.3) | 0 | 0 | 0 |
| No response | 2 (5.0) | 0 | 0 | 0 | 0 | 0 |
| NA/missing | 18 (45.0) | 1 (6.7) | 6 (37.5) | 6 (24.0) | 7 (38.9) | 5 (38.5) |
| 12 months | ||||||
| CR | 15 (37.5) | 12 (80.0) | 5 (31.3) | 15 (60.0) | 8 (44.4) | 5 (38.5) |
| VGPR | 1 (2.5) | 1 (6.7) | 1 (6.3) | 0 | 0 | 0 |
| PR | 0 | 1 (6.7) | 1 (6.3) | 0 | 0 | 0 |
| Mixed disease | 0 | 0 | 1 (6.3) | 1 (4.0) | 1 (5.6) | 0 |
| PD | 1 (2.5) | 0 | 0 | 0 | 0 | 0 |
| No response | 1 (2.5) | 0 | 0 | 0 | 0 | 0 |
| NA/missing | 22 (55.0) | 1 (6.7) | 8 (50.0) | 9 (36.0) | 9 (50.0) | 8 (61.5) |
| Developed cGVHD, n (%) | ||||||
| Yes | 11 (27.5) | 7 (46.7) | 6 (37.5) | 10 (40.0) | 9 (50.0) | 3 (23.1) |
| NA/missing | 4 (10.0) | 0 | 0 | 0 | 1 (5.6) | 3 (23.1) |
| aGVHD recurrence, n (%) | ||||||
| Yes | 15 (37.5) | 2 (13.3) | 4 (25.0) | 5 (20.0) | 6 (33.3) | 3 (23.1) |
| NA/missing | 0 | 1 (6.7) | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michonneau, D.; Devillier, R.; Keränen, M.; Rubio, M.T.; Nicklasson, M.; Labussière-Wallet, H.; Carre, M.; Huynh, A.; Viayna, E.; Roset, M.; et al. Treatment Patterns and Clinical Outcomes of Patients with Moderate to Severe Acute Graft-Versus-Host Disease: A Multicenter Chart Review Study. Hematol. Rep. 2024, 16, 283-294. https://doi.org/10.3390/hematolrep16020028
Michonneau D, Devillier R, Keränen M, Rubio MT, Nicklasson M, Labussière-Wallet H, Carre M, Huynh A, Viayna E, Roset M, et al. Treatment Patterns and Clinical Outcomes of Patients with Moderate to Severe Acute Graft-Versus-Host Disease: A Multicenter Chart Review Study. Hematology Reports. 2024; 16(2):283-294. https://doi.org/10.3390/hematolrep16020028
Chicago/Turabian StyleMichonneau, David, Raynier Devillier, Mikko Keränen, Marie Thérèse Rubio, Malin Nicklasson, Hélène Labussière-Wallet, Martin Carre, Anne Huynh, Elisabet Viayna, Montserrat Roset, and et al. 2024. "Treatment Patterns and Clinical Outcomes of Patients with Moderate to Severe Acute Graft-Versus-Host Disease: A Multicenter Chart Review Study" Hematology Reports 16, no. 2: 283-294. https://doi.org/10.3390/hematolrep16020028
APA StyleMichonneau, D., Devillier, R., Keränen, M., Rubio, M. T., Nicklasson, M., Labussière-Wallet, H., Carre, M., Huynh, A., Viayna, E., Roset, M., Finzi, J., Pfeiffer, M., Thunström, D., Lara, N., Sabatelli, L., Chevallier, P., & Itälä-Remes, M. (2024). Treatment Patterns and Clinical Outcomes of Patients with Moderate to Severe Acute Graft-Versus-Host Disease: A Multicenter Chart Review Study. Hematology Reports, 16(2), 283-294. https://doi.org/10.3390/hematolrep16020028







